Drive progress in plasma science by promoting new knowledge and research capabilities, guided by a robust ethical approach and utmost respect for human rights.
Our roadmap. Grifols 2030 Agenda
- Promote internal and external, plasma and non-plasma projects in key therapeutic areas
Priorities
-
ACCELERATE PROGRESS
- New therapies, products and solutions
- Improvements and new indications for existing products
-
SUPPORT
- Healthcare systems
- Competitiveness
-
COOPERATE
- Support scientific cooperation, education and research capabilities to drive progress in scientific knowledge
-
OPTIMIZE
- Achieve greater efficiencies
- Improve in-house productivity

First-place recipient of the Gartner Eye on Innovation Awards 2022 in the “Healthcare and Life Sciences” category
Main therapeutic areas + diagnostic
-
 Immunology
Immunology
-
 Neurology
Neurology
-
 Infectious diseases
Infectious diseases
-
 Pulmonology
Pulmonology
-
 Hepatology & Intensive Care
Hepatology & Intensive Care
-
 Hematology
Hematology
-
 Other therapeutic opportunities
Other therapeutic opportunities
-
 Diagnostic
Diagnostic



A robust innovation ecosystem
Grifols promotes scientific advances in line with its overriding mission to enhance people’s health and well-being. The company encourages research cooperation and competencies across several fronts, including in-house initiatives, investee collaborations, public-private partnerships and financial contributions to third-party programs. At the same time, it works continually to optimize the efficiency and productivity of its internal systems.
3 core objectives in 2023
- Accelerate and prioritize projects
- Optimize the innovation infrastructure
- Forge new innovation models
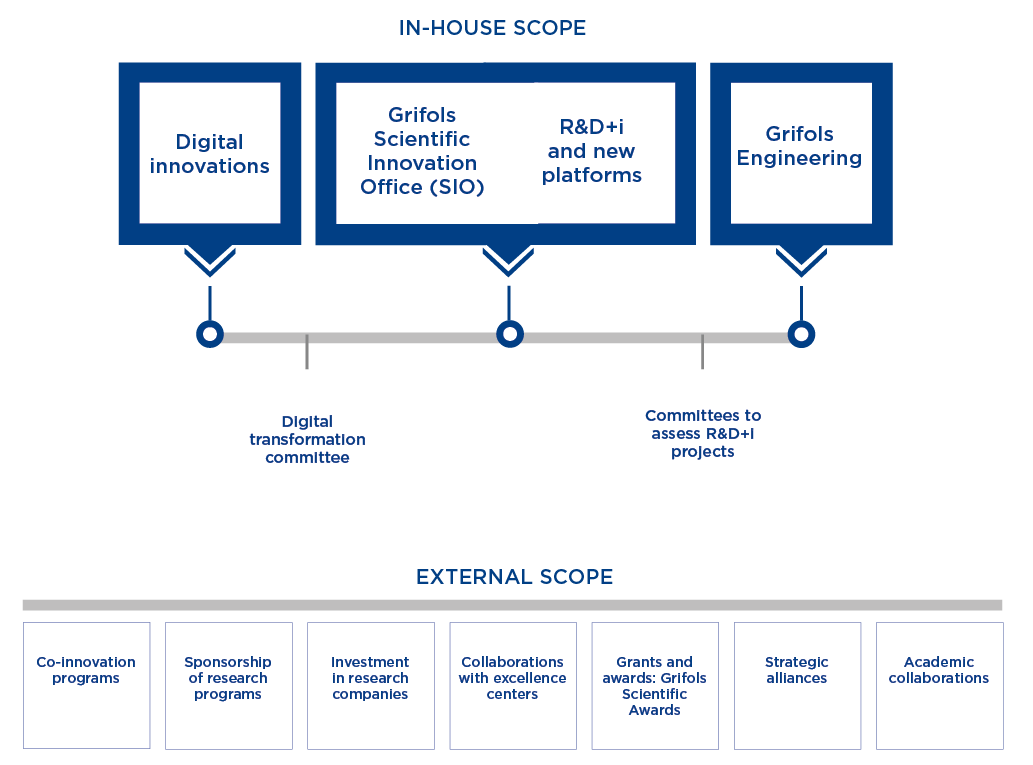
The company manages its R&D+i aimed at discovering new treatments through the Grifols Scientific Innovation Office (SIO). In 2023, these functions were restructured and organized to prioritize Grifols’ core strategic projects.
New leadership
Scientific Innovation Office 2023
Greater efficiency
- Ongoing review of progress and opportunities
- Focus on quality control
- Two-tier approach
Results oriented
- Promotion of Biotest projects
Centralized and global
- Led by new Chief Scientific Innovation Officer (CSIO)
- Discovery Plasma
- Discovery Recombinant
- Drug Development
- External Innovation
- Scientific & Medical Affairs
- Global Intellectual Property
- Scientific Business Development
- Controlling
- Project Management Office & Strategy
Resources allocated to R&D+i
R&D+i INVESTMENTS
395M
6% share of revenues
€1,682M+ invested over the last five years
RESOURCES
1,260+
people dedicated to R&D+i
90+ external researchers
PATENTS
2,705
patents
858 patent applications
1,283 patents that expire in the next 10 years
Research lines
- Plasma proteomics, fractionation and purification
- Single-cell transcriptomics
- Machine learning AI platform for target discovery
- Neuronal functional assay platform
- Therapeutic target selection and validation
- Polyclonal recombinant expression and manufacturing
- Mammalian cell line for site-directed integration
- Platform for discovery monoclonal antibodies
Investee companiess
- Araclon -Spain: Specialized in the research and development of new treatments and diagnostic tests for Alzheimer’s disease.
*This data includes Biotest.
Ethics, science and innovation
For Grifols, advances in life sciences should never be severed from their intrinsic humanistic component, emerging always from an ethical and social construct. The Víctor Grífols Lucas Foundation is the entity that translates this firm commitment into action.
Grifols SIO committees supervise and monitor of all issues related to clinical trials, including their ethical ramifications.
In this regard, the company subscribes to three fundamental and universal principles, which together govern the ethics of its clinical trials as defined in its Human Rights Policy.
We subscribe to three fundamental and universal principles
Respect for people
Respect for an individual’s ability to make decisions freely and independently, and protection of at-risk groups of people who participate as research subjects. This principle is expressed through informed consent forms.
Welfare
Guarantee the health of people who participate in clinical trials. Risks must be minimized and benefits maximized for all participants. For Grifols, protecting people’s health takes precedence over professional and personal interests, research advances and the search for knowledge.
Justice
Research must strike a balance between benefits and risks. All subjects must be treated with equal consideration and there must be no discrimination in the selection of subjects. Under this principle, participants are never exposed to unsafe situations to benefit another person. There is an obligation to safeguard the rights of vulnerable groups.
Grifols’ Human Rights Policy is available on its corporate website
Our commitments
Clinical trials
Grifols is committed to protecting the rights, safety and well-being of patients who take part in the clinical trials it conducts or sponsors. All clinical research led by Grifols or on its behalf adheres to the standards defined in the International Conference on Harmonization of Good Clinical Practice (ICH GCP); the protection of human beings under the Declaration of Helsinki (1964); and applicable local laws and regulations.
Clinical trials are described in a detailed protocol and evaluated by regulatory authorities and external ethics committees. They only begin once a favorable decision has been handed down.
Participants submit a written, signed and dated informed consent form. The lead researcher (or assigned healthcare professional) provides appropriate information, resolves any doubts and gives potential clinical-trial subjects sufficient time to make an informed decision on their participation.
To maintain quality control, Grifols has standard operating procedures that guarantee the proper execution of its clinical trials and documentation of their related trial data according to protocol, ICH GCP principles and applicable regulatory requirements. In addition, Grifols has detection procedures that enable its clinical professionals to detect and document possible fraud or misconduct in clinical investigations.
The company has several measures to ensure the transparency of data collected in its clinical trials, as well as protecting subjects’ anonymity and personal data. Grifols also subscribes to the principles of the codes of conduct regulating the processing of personal data from clinical trials and other applicable clinical and pharmacovigilance research.
Additional information on the protocol, status and results stemming from Grifols’ clinical trials are disclosed on publicly accessible registries, including www.clinicaltrials.gov and the EudraCT website, which records findings of clinical trials carried out under the European Medicines Agency (EMA). The findings of many of Grifols’ clinical trials are shared in international conferences and scientific journals.
Responsible testing
Grifols is committed to the responsible use of laboratory animals when required for the development of new life-sustaining therapies.
Whether studies are carried out in university settings or in external laboratories, Grifols researchers work closely with regulatory agencies and the Institutional Animal Care and Use Committee (IACUC) to ensure the safe and ethical treatment of animals.
All facilities are approved by the competent authorities where research is conducted. In the U.S., Grifols facilities are certified by the Association for Assessment and Accreditation of Laboratory Animal Care or equivalent organizations, and hold the highest accreditation possible for animal-testing laboratories.
In Europe, all laboratories comply with Directive 2010/63/EU relating to protecting animals used for scientific purposes and undergo country-specific inspections by country-specific authorities.
Grifols research adheres to the “Alternatives and the 3Rs” (Replacement, Reduction and Refinement) protocol, which advocates (i) Replacing the use of animal-testing for alternative techniques or avoiding it completely; (ii) Reducing the number of animals used; and (iii) Refining how experiments are performed to ensure animals suffer as little as possible.
Treatment innovations
6 core therapeutic areas
| Pre-clinical | Phase 1 | Phase 2 | Phase 3 | Phase 4 / Regulatory | LCM | ||
| Immunology | recIG – IDP | ||||||
| Xembify® – CLL | |||||||
| Xembify® – Biweekly dosing - PID | |||||||
| Xembify® – Pre-filled syringes | |||||||
| Yimmugo® (IVIG NextGen) – PID |
** | ||||||
| Hepatology / Intensive case | Albumin-20% - Cirrhosis - PRECIOSA | ||||||
| Albumin-5% - Acute on chronic liver disease – APACHE | |||||||
| FlexBag® (U.S., EU) | |||||||
| Pulmonology | Alpha-1 AT in non-cystic fibrosis bronchiectasis | ||||||
| Alpha-1 AT 15% (SC) – AADT | |||||||
| Prolastin-C® - AADT - SPARTA | |||||||
| Prolastin® vials 4-5 g. (EU) | |||||||
| Hematology | ATIII – Sepsis1 | ||||||
| Fibrinogen - Cong. deficiency & severe hypofibrinogen |
|||||||
| Fibrinogen – Acquired deficiency |
|||||||
| Fostamatinib2 - ITP – Refractory patients | |||||||
| Yimmugo® (IVIG NextGen) ITP |
** | ||||||
| Infectious diseases | GIGA 2339 - VHB | ||||||
| Trimodulin (IgM) – EScCAPE |
|||||||
| Cytotec® pregnancy – CMV infection |
|||||||
| Neurology | GRF6019 – Alzheimer’s | ||||||
| GRF6021 – Parkinson’s with dementia | |||||||
| Aβvac403 - Alzheimer’s | |||||||
| AKST4290 – Parkinson’s | |||||||
| AMBAR-Next – Alzheimer’s | |||||||
| Others | GIGA564 - Anti-CTLA-4 mAb Oncology | ||||||
| AKST4290 - Neovascular age-related macular degeneration (AMD) | |||||||
| VISTASEAL™ (fibrin sealant) - Biosurgery pediatric use | |||||||
| OSIG – Dry eye disease |
1 Association with Endpoint Health; 2 Rights licensed by Rigel Pharmaceuticals in the EU and other countries; 3 Project led by Araclon (Grifols investee).
** Commercialization started.
Projects ![]() Biotest
Biotest
More information on Grifols research pipeline: https://www.grifols.com/es/key-therapeutic-areas
Maximizing Biotest’s full potential
In 2023, Grifols continued to promote Biotest’s R&D projects that expand and enrich its innovation portfolio, and support its aim of increasing the availability of plasma therapies for patients worldwide.
Core projects in the pipeline
Milestones and advances in 2023
- Completion of recruitment and treatment of 200 patients for the Phase 3 AdFirst study with fibrinogen. Findings are expected to be presented in 2024.
- First patient with severe community-acquired pneumonia (sCAP) treated with Trimodulin in the Phase 3 of the EScCAPE clinical trial, expected to enroll 590 adult patients from up to 20 countries. The EScCAPE study will test whether mortality is reduced in Trimodulin-treated sCAP patients following the promising results of the Phase 2 CIGMA clinical trial of sCAP patients with invasive mechanical ventilation treated with Trimodulin.
- Expansion of the TRICOVID trial for patients with community-acquired pneumonia (CAP) The Phase 3 TRICOVID (Trimodulin against COVID-19) trial analyzes the impact of Trimodulin as adjunctive therapy in over 330 hospitalized adult patients with moderate to severe COVID-19. This research will assess whether Trimodulin is effective in activating a broad spectrum of antibodies against bacteria, fungi, viruses and other pathogens that may lead to lung infections.
- First shingles patient treated with the herpes zoster virus-specific hyperimmunoglobulin Varitect® CP (VZV-IG) as part of the prospective, multicenter, observational VARIZOSTA study. This study, comprised by 160 subjects from 15 German centers, aims to expand data on the efficacy and safety of routine use of Varitect® CP in patients with complex herpes zoster compared to standard therapy.
- FDA accepts marketing authorization application for Yimmugo®, Biotest’s IgG Next Generation, marking an important step in its U.S. market approval process. The application covers the indication primary immunodeficiencies (PID), with plans to expand it to include chronic primary immune thrombocytopenia (ITP) after receiving this initial clearance. The FDA’s decision is expected in June 2024.
- Yimmugo® receives clearance in the United Kingdom for the treatment of patients with congenital and acquired immunodeficiencies and for immunomodulation.
More details on Biotest’s research pipeline (biotest.com)
More information on Yimmugo
We promote wide-ranging in-house initiatives
Xembify® to prevent infections in CLL patients
linical trial for subcutaneous immunoglobulin Xembify® to help prevent infections in patients with secondary immunodeficient chronic lymphocytic leukemia (CLL), which affects more than 375,000 people in the U.S.
Phase 3 double-blind
clinical trial
380+ participants
100 Health Centers
First patient treated in 2023
This trial is being conducted in the U.S.
and Europe
Alpha-1 in pulmonary emphysema
SPARTA evaluates the efficacy and safety of two weekly intravenous alpha-1 dosing schedules in subjects with pulmonary emphysema caused by alpha-1 antitrypsin deficiency (AATD).
Phase 3/4 double-blind clinical trial
2 dosing regimen
60 and 120 weekly/mg/kg
Recruitment finalized with 339 patients in 2023
Albutein in decompensated cirrhosis
PRECIOSA clinical trial to evaluate the efficacy and safety of Albutein® in conjunction with standard medical therapy to increase survival in patients with decompensated cirrhosis and ascites awaiting transplantation.
Phase 3 clinical trial
69 participating centers
Recruitment finalized with
400 patients
Results in 2024
Milestones and advances in plasma therapies
- Encouraging findings from the Phase 4 XEMBIFY® study, which evaluated biweekly dosing of Grifols’ subcutaneous immunoglobulin (SCIV) at 20% concentration in patients with primary immunodeficiencies. The study showed similar safety and tolerability profiles between biweekly and weekly administrations. This will support FDA clearance of biweekly dosing, which is already approved in certain European markets. The FDA’s decision is expected by mid 2024.
- Global collaboration and licensing agreement with Selagine, a company dedicated to developing novel therapeutics for ocular diseases, to explore the potential of immunoglobulin (Ig) eye drops to treat dry eye disease, known to affect more than 100 million people globally.
- Grifols meets enrollment target of 339 patients for SPARTA (Study of ProlAstin-c Randomized Therapy with Alpha-1 augmentation), a phase 3 clinical study to assess if alpha-1-antitrypsin deficiency (DAAT) patients with emphysema have a slower disease progression if treated with two separate weekly doses of Prolastin®-C. The study will move onto the next stage, with core findings expected in 2026.
- Completion of Prolastin 4-5g (alpha-1) project, which will enable 2024 launch of a more convenient presentation of this plasma treatment in several European markets, in benefit of both patients and healthcare professionals.
- Positive topline results from phase 3b study of its fibrin sealant to treat surgical bleeding in pediatric patients. Known commercially as VISTASEAL™ in the U.S. and VERASEAL™ in Europe, this sealant combines two plasma proteins (fibrinogen and thrombin), and is applied with an airless spray technology to rapidly form clots. Grifols’ fibrin sealant is marketed and distributed by Ethicon, a Johnson & Johnson MedTech company as part of the strategic collaboration between the two companies. Regulatory authorities are expected to rule in 2024.
- BMC Neurology publishes the results of the GAMEDIS study, which evaluated fatigue, depression and product tolerability during long-term treatment with intravenous immunoglobulin (Gamunex® 10%) in patients with chronic inflammatory demyelinating polyneuropathy (CIPD). GAMEDIS was a multi-center, prospective, non-interventional study of 148 adult CIDP patients in Germany, who were treated for a mean of 83 weeks. The study found the treatment to be safe and well-tolerated.
Main product launches
- Launch of XEMBIFY® in Spain, Australia and Wales (UK)
- Expansion of TAVLESSE® (fostamatinib) in Europe
- More markets for VISTASEAL™



More information on product launches: “Financial Performance”.
| R&D PROJECTS BASED ON THEIR DEVELOPMENT PHASE | |||
| 2023* | 2022* | 2021 | |
| Discovery | 24 | 19 | 21 |
| Pre-clinical | 23 | 28 | 30 |
| Clinical | 22 | 23 | 22 |
| Post-commercialization studies | 14 | 39 | 9 |
| Other projects | 16 | 14 | 14 |
| Total Biopharma R&D projects | 99 | 123 | 96 |
* Includes Grifols and Biotest.
Other initiatives in neurodegenerative diseases
ALKAHEST
Through its investee Alkahest, Grifols continues to drive new knowledge of the plasma proteome to determine plasma proteins associated with aging, a discovery that could extend its therapeutic benefit to other diseases, including those related to the central nervous system.
There are ongoing clinical programs with plasma fractions and small molecules in patients with Alzheimer’s disease, Parkinson’s disease and neovascular age-related macular degeneration (AMD).
Araclon and Alzheimer’s disease
Grifols became an Araclon Biotech shareholder in 2012. Since then, it has supported and promoted its consolidation as a pioneering developer ofprojects to diagnose and treat Alzheimer’s disease.
Results from phase 2 clinical study of ABvac40 Alzheimer’s vaccine
Positive results were reported in the phase 2 trial of ABvac40, an active vaccine against the Aβ40 peptide to treat patients with early-stage Alzheimer’s disease (AD). Findings show that ABvac40 had a favorable safety profile, elicited a robust immune response against Aβ40, and showed some potential cognitive benefits in early-stage AD patients, meeting primary endpoints and showing differences between the vaccine- and placebo-treated groups in some secondary exploratory endpoints.
ABvac40 is uniquely designed to target the C-terminal end of the Aβ40 peptide, believed to prevent harmful reactions and avoid immune triggers responsible for meningoencephalitis, a complication observed in earlier AD vaccines.
While the trial was not designed to find efficacy on neuropsychological scales, the ABvac40-treated group exhibited up to a 38% reduction in disease progression, as reflected by the Mini-Mental State Examination (MMSE) score. These findings suggest the potential efficacy of ABvac40 in addressing the cognitive decline associated with AD.
These clinical data were presented at various scientific conferences, including the 2023 European Alzheimer’s Disease Consortium, the CTAD 2023 Alzheimer’s Disease Clinical Trials Conference and the 75th Annual Meeting of the Spanish Neurology Society.
More details:
https://www.Araclon.com
Complete details on the phase 2 study on Abvac40
ABtest-MS to detect early-onset Alzheimer’s
Araclon’s ELISA Abtest-IA assays analyzed β-amyloid peptides in human plasma, proving their potential to help identify cognitively normal individuals with Alzheimer’s disease (AD)-related pathological changes in their brains. Following these positive findings, it developed ABtest-MS, an innovative assay to simultaneously determine total Aβ40 and Aβ42 levels in plasma by liquid chromatography tandem mass spectrometry.
In 2023, Alzheimer’s Research & Therapy magazine published findings on a trial on the effectiveness of ABtest-MS testing in detecting early AD disorders. A collaboration between ACE Alzheimer Center (Barcelona, Spain) and Samsung Medical Center (Seoul, South Korea), the study analyzed plasma samples from 200 people with subjective memory complaints and monitored them over a two-year timeframe.
The trial successfully identified individuals at higher risk of disease progression, confirming the robustness and utility of ABtest-MS demonstrated in previous studies as a potential pre-screening tool for clinical trials, prevention strategies and clinical practice.
GigaGen, non-plasma innovations
GigaGen is dedicated to the discovery and development of recombinant polyclonal antibody-based drugs to treat immunodeficiencies, infectious diseases and immunotherapy-resistant cancers. Its proprietary technology platforms advance the discovery of potent monoclonal antibody therapeutics and a new class of drugs: recombinant polyclonal antibodies.
Phase 1 clinical trial for GIGA-564, GigaGen’s first oncology drug candidate
In 2023, GigaGen received FDA clearance for an Investigational New Drug (IND) designation to start a phase 1 clinical trial to evaluate the company’s oncology candidate, GIGA-564, for the treatment of advanced solid tumors.
Scheduled to begin in 2024, the study will be led by researchers from the National Cancer Institute (U.S.) in close collaboration with GigaGen under their recently signed collaboration agreement.
Expansion of GigaGen’s research contract with the U.S. Department of Defense
GigaGen will collaborate with the U.S. Department of Defense to demonstrate the utility of its first-in-class recombinant human polyclonal antibody discovery platform against biological threats including botulinum neurotoxins (BoNT) A and B. The expanded agreement will facilitate further research on GigaGen’s next-generation platform capabilities to rapidly create synthetic human antibodies that surpass natural immune responses. The agreement’s value now stands at USD 11.8 million for transformative projects, including manufacturing support and novel studies to prove the increased potency of GigaGen’s BoNT hyperimmune product.
The contract expansion reaffirms the Department’s confidence in GigaGen’s technology and ability to develop key therapies against high-priority pathogens.
More information: GigaGen
Innovation in Diagnostics
Milestones and product launches in 2023
First IVDR certifications for class D Diagnostics products
Grifols received the first certifications for its class D Diagnostic products under the new European Union Regulation on In Vitro Diagnostic Medical Devices (IVDR). These include all red blood cell reagents and some of the gel cards, such as DG Gel ABO/Rh (2D) + Kell.
U.S. market launch: AlphaID™ At Home
Grifols launched AlphaID™ At Home Genetic Health Risk Service (AlphaID™ At Home) in the U.S. market in May 2023. This free service allows patients with chronic obstructive pulmonary disease (COPD) to detect their genetic risk of alpha-1 antitrypsin (alpha-1) deficiency through a small saliva sample, with no need to visit a healthcare professional. Alpha-1 affects an estimated one in every 2,500 Americans.
Innovation in creating the laboratory of the future
For the first time, the Procleix Panther System featuring ART technology was connected to a fully automated laboratory sample processing platform through a collaboration between Grifols Diagnostic and Lifeblood, the Australian Red Cross entity in charge of the collection, screening and distribution of the country’s blood and biological products. The automation of these processes enhances safety and quality, while offering future-forward insights for global laboratories.
New solution to facilitate pre-transfusion compatibility testing in multiple myeloma patients
In 2023, the company launched Grifols sCD38, the first soluble recombinant protein designed to block anti-CD38 antibodies in multiple myeloma patients treated with daratumumab. This innovation ensures the speed and accuracy of blood transfusion tests, critical for high-quality treatment.
Digital innovation
Digital innovation is a core hub in Grifols’ operations, allowing the company to detect market opportunities and better compete in today’s fast-paced business landscape. With the objective of exploring, and enhance digital tools that add value to the business model, the company continues to advance under the leadership of the Chief Digital Information Officer (CDIO).
In 2023, the company continued to advance in its digital transformation process by leveraging the knowledge and experience acquired since 2018 to spearhead a comprehensive redesign of its community and ecosystem, guided by a local approach with a global vision.
Grifols’ digital strategy is based on three key pillars:
1. Digital Boost: driving the implementation of innovative initiatives
2. Literacy and Spread: effective communication of core actions to proactively foster cultural change
3. Digital Networking & Open Innovation: encouraging open-mindedness to new ideas and cultivating an innovation-friendly environment
Grifols created “Digital Innovation Local Hubs” within each business unit to reinforce these core pillars. These hubs will serve as catalysts for cultural change, helping the company better address challenges and seize new opportunities.
This holistic strategy allows Grifols to drive innovation internally and boost its renown as a proactive agent in adopting new ideas and industry practices. Grifols advances these innovation efforts through collaborations with several external entities. In 2023, the company joined the Barcelona Health Hub (BHH), dedicated to fostering innovation and interaction in the digital health space. The BHH’s 350 members include startups, healthcare institutions, universities, large corporations and investors. This participation allows Grifols explore and fast-track the adopting of leading-edge digital health platforms and technologies.
Digital innovation: areas of impact
Comercial
Client
+ value
Industrial
Value chain and operations
+ optimization
Plasma
Donors
+ experience
+ efficiency
I+D
New sources of value
Quality
+ safety
Corporate
+ processes
+ employee experience
Harnessing the power of artificial intelligence
As a firm believer in the immense impact and business potential of artificial intelligence, Grifols continuously explores new AI solutions to maximize its manufacturing efficiency and sustainability, as well as enhance its R&D initiatives and other strategic areas. The main projects of 2023 have been:
AI systems to optimize industrial energy consumption
Grifols rolled out an AI application in its cooling system to monitor internal and external parameters and discover patterns to discern the optimal time to activate the system. Armed with this information, the company can perform a smoother start-up, leading to lower energy consumption.
Grifols began exploring AI solutions in 2021 with the aim of optimizing and improving its industrial energy consumption. The significant energy savings recorded in 2022 and 2023 moves Grifols closer to its objective of improving its industrial energy efficiency by 15% by 2030.
The company intends to build on this initiative and achieve an even better energy-management system by incorporating digital-twin technology in its manufacturing operations.
AI implementation in immunoglobulins production
Grifols implemented AI platforms in its Biopharma plants with the aim of optimizing its intravenous immunoglobulin (IVIg) manufacturing performance. These systems collect data from production processes, identify critical parameters and learn how variations affect the amount of protein obtained. Based on this information, the platform proposes new thresholds to achieve higher IVIg yields.
Grifols implemented AI platforms in its Biopharma plants with the aim of optimizing its intravenous immunoglobulin (IVIg) manufacturing performance. These systems collect data from production processes, identify critical parameters and learn how variations affect the amount of protein obtained. Based on this information, the platform proposes new thresholds to achieve higher IVIg yields.
Launched in 2022 through the Scientific Innovation Office, Grifols Innovation with Google Academy (GIGA) promotes innovation by fostering an organization-wide digital culture and mindset. Under this umbrella, Grifols will work together with Google to implement 12 AI-driven innovations aimed at accelerating and streamlining its R&D processes. These initiatives are expected to yield promising returns on investment and benefit numerous corporate areas, including Clinical Trials, Medical Affairs, Data Discovery, Drug Discovery and Biopharmaceutical Therapies.
Manufacturing innovation
Grifols works to advance the efficiency and sustainability of its production processes in line with its growth strategy. Leveraging its in-house engineering expertise and collaborations with other institutions and organizations, it continuously explores options to integrate new technologies, automated systems, digitalization opportunities, AI and new materials. The following were among its core projects in 2023.
Virtual modeling of process bioreactors to boost plasma protein yields
In collaboration with the Barcelona Super Computing Center, Grifols is working to model the reactors used in the precipitation of the diverse protein fractions1, with the aim of improving the purity of the paste per fraction and achieving higher plasma-protein yields.
Development of a new sterile filling machine
In the production of biological drugs, maintaining sterile conditions for the dosing and filling phase is critical. While considered a global industry standard, Grifols’ system was initially designed to process small formats of up to 100 milliliters, or large formats of up to 500 milliliters. The new machine processes formats from 50 ml to 400 milliliters, offering greater flexibility.
Optimizing plasma logistics operations with SAS
Grifols developed and implemented a Supervised Aggregation System (SAS) to incorporate RFID (radio frequency identification) technology into its clients’ plasma logistics operations. This system, fully integrated with the customer’s donor database, improves traceability and reduces operating costs by enabling real-time wireless readings in its logistics operations.
1. Fractionation is the process of separating proteins from human plasma. In the blood products industry, Cohn fractionation is the most widely used, entailing the precipitation and subsequent separation of pastes rich in different protein groups (fractions).
Research collaborations and support
Sponsorship of ISR Program
Grifols’ Investigator-Sponsored Research (ISR) advances scientific knowledge of plasma proteins by supporting pre-clinical and clinical research.
$7.5M
allocated to research over the past 5 years to complement public-sector investments
Grifols Scientific Awards and research grants
These distinctions recognize innovative proposals developed to enhance people’s health, well-being and quality of life.
4.7M
over the last 5 years toward scientific awards and research grants
Scientific journal specialized in plasma
Grifols was a key contributor in creating Plasmatology, the first scientific journal dedicated to plasma science. This trailblazing publication aims to become a global industry reference by featuring the most relevant and rigorous research, from basic research to clinical applications. The journal has open access and indexed in a range of scientific databases.
34
articles published since its March 2021 launch
Grifols Chair for the Study of Cirrhosis and Albumin
Grifols created the Grifols Chair for the Study of Cirrhosis in 2015 to promote research and awareness of liver disease, with an emphasis on cirrhosis. A private initiative with a global reach, the Chair forms part of the European Foundation for the Study of Chronic Liver Failure (EF-Clif). Prof. Vicente Arroyo serves as the president of EF-Clif and holder of the Chair, whose executive board includes a Grifols representative.
14M
invested over the last 5 years in liver disease research
