Annex I. Bases for the preparation: scope and methodology – Total Tax Contribution
Purpose and scope
The “Fiscal Contribution” section included in the “Financial Performance” chapter provides information on the taxes paid by the Grifols Group globally in 2023 in a clear and concise manner. Disclosures includes data from the following territories: Spain, the United States, Ireland, Germany and United Kingdom, as the most relevant in terms of their business volume and presence within the Grifols Group.
The measurement used data obtained from information systems following the PwC Total Tax Contribution (TTC) methodology. In addition to the amounts indicated, other tax payments may have been omitted because they are not individually identified in the information systems and/or are not significant in terms of materiality.
TTC methodology
The Total Tax Contribution methodology measures the total impact of a company’s tax payments. This assessment is made from the perspective of total tax contributions paid directly to the different public administrations as a result of the Grifols Group’s economic activity.
In general, the TTC methodology allocates both input and output taxes to each tax year on a cash basis.
The following points should be kept in mind regarding this methodology:
1. It distinguishes between taxes that are a cost to Grifols and taxes collected.
Taxes borne are taxes paid by Grifols to the governments of countries in which it operates. These taxes represent an effective cost for Grifols, such as taxes on profits and certain environmental taxes.
The taxes collected are those that have been received as a result of Grifols’ economic activity, without representing a cost to the Group other than that of its management. These include withholdings from workers due to income tax, VAT, and other taxes on products and services. Nonetheless, these amounts are paid into public coffers as a result of Grifols’ economic activity and therefore should be included in the analysis since they represent tax revenue stemming from Grifols’ operations.
2. TTC framework classifies taxes under 5 categories for clarification purposes:
(i) Profit taxes: taxes borne on profits earned by companies such as corporate income tax, business tax and taxes levied as withholding taxes on payments to third parties.
(ii) Property taxes: taxes on the ownership, sale, transfer or occupancy of property.
(iii) People (or Employment Taxes): employment-related taxes both borne and collected, including employee income tax withholdings and social security payments payable by both Grifols and the employee.
(iv) Taxes on Products and Services: indirect taxes on the production and consumption of goods and services, including VAT and customs duties.
(v) Planet (Environmental Taxes): taxes on the supply, use or consumption of products and services deemed to affect the environment.
3. It includes all tax payments made to public administrations
Readers should take into account that figures detailed in this report include tax payments made to public administrations for items whose characteristics make them tax-related, although they have not been classified as such for cyclical or historical reasons. Readers should also take into consideration that figures in this report exclude other amounts that, based on the methodology and reports issued by the OECD and other international administrations, are not considered a tax contribution.1
4. Profit before taxes assumptions made during the preparation of this report
The amount of profit before tax excludes intercompany dividends to avoid duplicating the same income of various entities in the case of its distribution as dividends to other Grifols entities. This calculation enables reflecting the objective amount of profit before taxes at country levels and calculating the objective ETRs, as dividends are usually subject to beneficial tax treatment compared to the other types of income (i.e. “participation exemption” regime).
5. There are certain particularities with regard to value added tax (VAT) and equivalent taxes
Value added tax (and equivalent taxes) is characterized as a tax on products and services collected, the amount of which reflects the result of net payments made by Grifols to the tax authorities in its jurisdictions of operation in the corresponding period.
In calculating VAT, the country-specific figure indicated for this concept includes the positive amount paid to the corresponding tax authorities, resulting from subtracting the VAT accrued from the amount of VAT deducted.
No figure shall be shown for this item in cases in which the net amount resulting from subtracting VAT accrued from VAT deducted for an entire year and country is negative due to a refund.
On the other hand, VAT amounts that are not refundable because the value chain cannot be continued by means of the reverse charge instrument shall be considered as input tax on products and services, since they represent a cost for the company.
1. Main sources of Total Tax Contribution Methodology:
Annex II. SROI – Social Return on Investment (SROI) methodology
The Social Return on Investment (SROI) method aims to gain a deeper understanding of an organization’s social, environmental and economic impact. The SROI method represents a valuable cost-benefit analysis, offering the leadership team and investors a solid decision-making tool to assess and optimize the firm’s social and environmental impacts.
The SROI uses individual assessments to measure the change in stakeholders’ lives because of Grifols’ activities. The evaluations are quantified and recorded on an impact map, and monetary value is then assigned to the resulting social, environmental and economic impacts.
2023 global SROI analysis
The study was conducted by Hugo Narrillos Roux, holder of a doctorate with honors in economics from the Complutense University of Madrid, a specialist in social value and the author of “Social Economy: Valuation and Measurement of Social Investment (SROI method) and his doctoral thesis, "The Social Return on Investment: A Good Method to Measure the Social Value Created by Companies".
Mr. Narrillos Roux is recognized as an accredited SROI professional by Social Value International, a network of professionals dedicated to generating knowledge on change and social value. He serves as a faculty member at several universities and a social-impact consultant at leading global organizations..
Main references:
Alpha 1: Augmentation Therapy for α1 Antitrypsin Deficiency: A Meta-Analysis. Chapman, K.R., Stockley, R.A., Dawkins, C., Wilkes, M. M., Navickis, R. J. COPD: Journal of Chronic Obstructive Pulmonary Disease, 6:177–184 (2009)
Factor VIII: Pasi, J., Hermans. C., Hakimi,Z., Nazir, J., Aballéa, S., Ezzalfani, M., and Fatoye, F. (2022): Improvement in pain-related quality of life in patients with hemophilia A treated with rFVIIIFc individualized prophylaxis: post hoc analysis from the A-LONG study. Therapeutic Advances in Hematology. 2022, Vol. 13: 1–9
PID: The impact of plasma-derived therapies in Europe. The health and economic case for ensuring sustainable supply. Copenhagen Economics. June 2021. Available at: https://www.copenhageneconomics.com/publications/publication/the-impact-of-plasma-derived-therapies-in-europe (website visited on January 2024).
CIDP: Hartung, H., Mallick, R., Bril, V., Lewis, R. A., Sobue, G., … Lawo, J. (2019). Patient-reported outcomes with subcutaneous immunoglobulin in chronic inflammatory demyelinating polyneuropathy: the PATH Study. European Journal of Neurology.
SID: Benbrahim, O., Viallard, J.-F., Choquet, S., Royer, B., Bauduer, F., Decaux, O., … Lévy, V. (2018). The use of octagam and gammanorm in immunodeficiency associated with hematological malignancies: a prospective study from 21 French hematology departments. Hematology, 24(1), 173–182.
ITP: Efficacy and mechanism of intravenous immunoglobulin treatment for immune thrombocytopenia in adults. Ruqayyah J. Almizraq1, Donald R. Branch. Annals of Blood. Vol 6 (March 2021).
GBS: Immunoglobulin Use for the Management of Painful Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Z. Panagiotis, Liampas, A., Pozotou, T., Parperis, K., Artemiadis, A., Hadjigeorgiou, G. (2022). Pain And Therapy (2022). 11:1219–1227
MG: Porras L.D., Homedes C., Alberti M.A., Santamaria V.V., Casasnovas C. (2022).Quality of Life in Myasthenia Gravis and Correlation of MG-QOL15 with Other Functional Scales. Journal of Clinical Medicine. 2022; 11(8).
HC: Runken, M. C., Caraceni, P., Fernandez, J., Zipprich, A., Carlton, R., & Bunke, M. (2019). The cost-effectiveness of albumin in the treatment of decompensated cirrhosis in Germany, Italy, and Spain. Health Economics Review, 9(1).
Annex III. Methodology and calculation of the adjusted and unadjusted pay gap
The following groups have been excluded from the calculation:
- SELT
- Executive Chairperson
- Partial retirees
- Expatriates or displaced employees
- Employees of foundations
- Undeclared and non-binary employees
- The company Plasmavita Healthcare is not yet fully integrated into the systems and policies of Grifols at 100%.
In total, 19,660 employees have been included in the wage gap calculation, distributed by country as follows:
- EE.UU.: 13,852
- España:4,103
- Alemania:1,334
- Irlanda: 371
According to the Global Reporting Initiative standards, the unadjusted gender pay gap is the difference between the average salary of men and the average salary of women, calculated based on the average salary of men. For the purposes of this report, average salary is understood as the mean of the gross annual fixed salary at 100% employment.
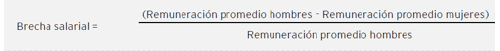
Unlike the unadjusted gender pay gap, adjusted wage gaps allow isolating the effect of wages from existing differences between men and women, considering both their socio-economic characteristics (age, seniority, level of education, etc.) and the positions they hold (working hours, type of occupation, etc.). In this way, adjusted wage gaps serve as a more reliable indicator to measure whether men and women receive "equal pay for equal work"
The existence of an unadjusted gender pay gap does not directly imply gender-based discrimination, as various factors affecting the compensation for a specific position must be considered, such as required experience, tenure in the role, responsibility, supervisory roles, shift work, and hardship, among others.
Therefore, the adjusted wage gap has been estimated using a multiple linear regression model that quantifies, through a single equation, the relationship between predictor or independent variables (Xi1,Xi2…XiM) and the dependent or response variable (Wi). This is done to better understand or explain the mechanisms of this relationship
In this equation, Wi represents the gross annual fixed salary at 100% employment for employee i transformed to its logarithm, while Gender is a dichotomous variable equal to 1 if male and 0 if female
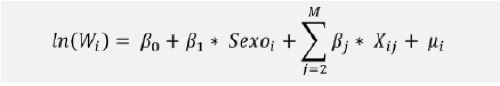
The econometric calculation of the adjusted wage gap has taken into account the following variables: Age, seniority, area, business division, professional category, performance rating, level of education, type of contract, Geodif, Plasma or non-plasma.
The results are presented for each country separately to avoid applying a currency exchange rate that may distort the outcome.
Due to confidentiality and personal data protection reasons, wage gap data is not shown for professional categories where there are fewer than 4 individuals of each gender.
In some cases with small groups, the adjusted wage gap data is not shown as it is not possible to obtain statistically significant results through the econometric model. For these cases, only the unadjusted wage gap data is presented.
Annex IV. Alliances and associations
- AECOC: Spanish Association of Manufacturers and Distributors.
- AENE: Spanish Association of Manufacturers and Distributors of Enteral Nutrition Products.
- AmCham: American Chamber of Commerce in Spain, China and Thailand.
- ASEBIO: Spanish Association of Bio Companies.
- BIOcom Life Sciences Organization of California: California association of bioscience companies and research institutes.
- Biotechnology Innovation Organization (BIO): the world’s premier biotech trade association whose membership includes industry firms, academic institutions and U.S. state-level centers and organizations.
- CAEME: Argentine Association for Pharmaceutical and Biotech Products.
- CBDL: Brazilian Chamber of In Vitro Diagnostics Companies .
- EMIG: Ethical Medicines Industry Group .
- EUCOPE: trade association representing small- to medium-sized pharmaceutical and med-tech firms in Europe .
- EURORDIS: non-governmental patient-driven alliance representing 949 rare disease patient organizations in 73 countries.
- Farmafluid: Spanish Association of Fluid Therapy and Parenteral Nutrition Pharmaceutical Laboratories .
- Farmindustria: Italian Association of Pharmaceutical Companies .
- Global Business Alliance: an association of globally focused U.S. firms that promotes foreign investment in the country.
- JACRI: Japanese Association of Clinical Reagents Industry .
- LEEM: French industry association representing drug companies operating in France.
- MedTech Europe: Trade association representing the medical technology industries, manufacturers of in vitro diagnostics and medical devices operating in Europe and diverse national associations.
- National Health Council (EE.UU.): platform for diverse organizations to forge consensus and drive patient-centered health policy.
- North Carolina BIO: trade association for North Carolina’s life science industry whose membership includes companies and research institutions working in the pharmaceutical, medical device, diagnostic, clinical research and agricultural biotechnology sectors.
- Pathology Technology Australia: Australian association of manufacturers and distributors of in vitro diagnostic reagents and systems.
- PPTA: Plasma Protein Therapeutics Association.
- SIGRE: not-for-profit organization established to ensure proper environmental management of medicines and their packaging in the home.
- SINDUSFARMA: Brazilian Association of Pharmaceutical Companies.
- United States-Spain Council: An organization of U.S. and Spanish leaders who work to cultivate stronger ties between both countries.
Grifols advances social progress by collaborating with an array of public- and private-sector organizations. The following table outlines its most significant financial contributions by industry:
| Activity | Involvement / commitment | 2023 contribution |
| PLASMA INDUSTRY | Grifols supports various projects related to the plasma industry, including the joint promotion of a global code of conduct, educational campaigns, access to clinical treatments, procurement of plasma as a raw material, and awareness campaigns on rare diseases. | 2,136,677 € |
| PHARMACEUTICAL INDUSTRY | Defense of policies and practices to promote the discovery of and access to life-enhancing medicines and vaccines for people around the world. Efforts to reinforce regulatory systems to ensure maximum safety throughout the value chain, from production to patient administration while acting ethically and professionally in alignment with Grifols Codes of Conduct.(1)(1) | 236,517 € |
| MED-TECH INDUSTRY | Efforts to highlight the social value and contribution of medical technologies, facilitating their access to patients, healthcare professionals, operators and healthcare systems. Promotion of value-based innovation to create more sustainable healthcare systems and meet the growing needs and expectations of health and medical-care systems. Adherence to the highest ethical standards for all training initiatives and interactions with healthcare professionals.(2) | 103,565 € |
| BIOTECHNOLOGY INDUSTRY | Participation in national non-profit associations of several bio-tech firms, aimed at increasing their social awareness and promoting innovation by advocating for public policies that favor the growth of this essential industry.(3) | 161,889 € |
(1) IFPMA - Homepage: (https://www.ifpma.org/
(2) Medtech Europe – Homepage (https://www.medtecheurope.org/)
(3) ICBA – Homepage (https://internationalbiotech.org/about/)
Annex V. Environmentally Sustainable Activities. Grifols’ activities under European Taxonomy
European Union Taxonomy
One of the cornerstones of the European Union’s Sustainable Finance Action Plan (SFAP) consisted of approving the EU Regulation 2020/8521 (known as the Taxonomy Regulation) in 2020. European Taxonomy comprises a classification system devised by the European Union to evaluate whether an economic activity can be considered environmentally sustainable, thereby guiding the degree of environmental sustainability of an investment. Fundamentally, European Taxonomy aims to establish a common and transparent framework for companies and investors to assess and report the environmental impact of their business activities. Its overarching goal is to channel investments towards sustainable activities and support the shift towards a more sustainable, greener economy.
The Taxonomy addresses different economic activities and industries providing uniform criteria to evaluate their contribution toward six defined environmental objectives:
- Climate change mitigation.
- Climate change adaptation.
- Sustainable use and protection of water and marine resources.
- Transition to a circular economy.
- Pollution prevention and control.
- Protection and restoration of biodiversity and ecosystems.
These environmental objectives defined by the European Union are detailed in the Delegated Regulation (EU) 2021/213982 published on June 4, 2021. This regulation includes a comprehensive list of economic activities deemed environmentally sustainable. For each activity, the regulation provides a description and specifies the technical criteria required to determine whether it contributes significantly to any of the six environmental objectives. Additionally, it outlines the criteria to ensure that the activity is carried out in such a way that it does not harm any other environmental objectives. Furthermore, in order to align with the European Taxonomy and be considered as sustainable, organizations must adhere to minimum social safeguards.
The European Taxonomy has established three key indicators based on specific financial ratios derived from data on CapEx investments, OpEx operating expenses and turnover linked to the activity carried out by the company.3
In this context, Grifols performed a comprehensive analysis of its economic activities along these three dimensions and their intersection with its six environmental objectives in order to determine which actions make a substantial contribution. The results were as follows:
| Turnover | CapEx | OpEx | |
| Eligibility in figures (€) | 5,096,622,614.77 | 132,070,212.93 | 45,300,275.21 |
| % Eligibility | 83.14% | 82.42% | 24.83% |
| Alignment in figures (€) | 0 | 12,760.39 | 0 |
| % Alignment | 0% | 0.008% | 0% |
This analysis was conducted in two phases: The first identified whether any of the economic activities associated with the three KPIs correspond to any of the activities outlined in the Taxonomy (eligibility assessment). The second assessed the extent to which these activities align with environmentally sustainable criteria (alignment).
First phase: Eligibility Analysis
This first phase involved assessing Grifols’ economic indicators (CapEx, OpEx and turnover) to identify whether the company’s activities correspond to the economic activities described in the Taxonomy Regulation on environmental objectives.
Unlike the previous two years, when the Taxonomy analysis was conducted solely in relation to the first two environmental objectives in which Grifols only reported activities linked to its investments or expenses incurred, this year marks a significant change. Grifols’ primary activity, and part of the company’s income, are included in the list of activities recognized by the Taxonomy. Specifically, this is “1.2. Manufacture of pharmaceuticals,” which falls under the environmental objective "Pollution Prevention and Control."
1. Regulation (EU) 2020/852 of the European Parliament and of the Council
2. Commission Delegated Regulation (EU) 2021/2139 of 4 June 2021 supplementing Regulation
(EU) 2020/852 of the European Parliament and of the Council and establishing the technical
selection criteria to determine the conditions under which an economic activity is deemed to
make a substantial contribution to climate change mitigation or adaptation, and to determine
whether that economic activity causes significant harm to any of the other environmental
objectives (europa.eu
3. The Taxonomy Regulation sets out specific criteria for using figures related to CapEx,
and turnover, which diverge from traditional accounting concepts. Therefore, there may be
discrepancies between the figures used to calculate the Taxonomy and those presented in other
sections of Grifols’ report.
Additionally, some of the company’s other economic activities have been identified, which while not directly related to its main activity, are related to the investments or expenses incurred, and considered eligible under the Taxonomy.
List of Grifols’ eligible activities for 2023
| Objective | Activity | Brief description in accordance to the Regulation | Brief description in accordance to Grifols’ activities |
| Pollution prevention and contro | 1.2 Manufacture of pharmaceuticals | Manufacture of pharmaceuticals | Grifols main activity, which consists of researching, developing, producing and marketing plasma-derive |
| Biodiversity | 1.1 Conservation, including restoration, of habitats, ecosystems and species | Initiation, development and realisation on own account or on a fee or contract basis, of conservation activities, including restoration activities, aimed at maintaining or improving the status and trends of terrestrial, freshwater and marine habitats, ecosystems and populations of related fauna and flora species. | In the town of Clayton, USA, Grifols has more than 120 hectares of protected forest, located in the vicinity of Grifols' production facilities. This environment includes eight hiking trails and have more than 300 species of plants and animals. The main objective of this project is to protect a large area of forest as a habitat for wildlife. |
| Climate Change Mitigationo | 7.3 Installation, maintenance and repair of energy-efficient equipment | Individual renovation measures to install, maintain or repair energy-efficient equipment. | As part of Grifols’ energy saving programme, the Erfurt
donation centre has switched from conventional lighting to
LED lighting. In addition, as a contribution to the objectives of Grifols’ Sustainability Commitment, the Haema AG donation centre in Germany has installed a green roof covering which mainly provides greater energy savings and thermal insulation. |
| 7.4 Installation, maintenance and repair of charging stations for electric vehicles in buildings (and in parking spaces adjacent to buildings) | Installation, maintenance and repair of charging stations for electric vehicles in buildings and in parking spaces adjacent to buildings. | Grifols facilita y promueve soluciones de movilidad más sostenibles, habilitando infraestructuras para la recarga eléctrica de vehículos en los aparcamientos de los empleados de Grifols de las oficinas de Sant Cugat. Este año, se ha efectuado una reubicación de todos los cargadores de vehículo eléctrico ubicados en aparcamientos subterráneos a zonas de aparcamiento exterior. |
Second phase: Alignment Analysis
In line with the requirements set out in the Taxonomy Regulation for the 2023 fiscal year, Grifols’ analysis specifically focused on those activities that contribute to the first two environmental objectives: Climate Change Mitigation and Climate Change Adaptation. This assessment was carried out based on the three essential conditions that an economic activity must satisfy to be classified as environmentally sustainable:
- Offer substantial contribution to at least one of the 6 objectives defined by the Taxonomy.(EU Regulation 2020/852 Arts. 10 to 16)
- Do no significant harm to the other defined objectives.(EU Regulation 2020/852 Art. 17))
- Comply with minimum social safeguards (EU Regulation 2020/852 Art. 18)
The results of the alignment analysis determine that Grifols contributes via investments to the objective of Climate Change mitigation. The following activities are considered to be classifiable as environmentally sustainable:
- 7.4 Installation, maintenance and repair of electric vehicle charging stations in buildings (and parking spaces adjacent to buildings).
In the alignment analysis of Grifols’ economic activities for the 2023 fiscal year, the aforementioned activities are deemed environmentally sustainable based on their substantial contribution to the “climate change mitigation” objective. These activities meet the technical criteria for substantial contribution and adhere to the principle of Do Not Significant Harm (DNSH) to other environmental objectives defined in the European Taxonomy. Additionally, they comply with the minimum social safeguards. The ways in which these activities meet each of the three conditions are detailed below:
Technical criteria for substantial contribution to climate change mitigation
Grifols is considered to contribute to the Climate Change Mitigation objective in the following economic activities, 7.4 Installation, maintenance and repair of charging stations for electric vehicles in buildings, since these activities meet the technical criteria of substantial contribution to the environmental objectives.
Do No Significant Harm (DNSH
Parallel to this, in the "Environment" section of this document, it was determined that Grifols’ economic activities adhere to the “Do No Significant Harm” technical criteria. Specifically, for these activities, the only criterion to be analyzed comes under the “Climate Change Adaptation” objective, as the regulation itself states that no significant harm must be caused to any of the other four objectives.
Minimum Social Safeguards
Finally, these activities are considered to be in alignment with environmentally sustainable criteria, since the sections on “Promoting Integrity” and “Human Rights” in this document determine that Grifols complies with the “Minimum Social Safeguards” set out in Article 18 of the Taxonomy Regulation.
Calculation of Economic Indicators
To illustrate Grifols’ contribution to sustainability, the following shows the percentages representing the proportion of the company’s total turnover, CapEx and OpEx which are attributable to eligible and/or aligned activities. This offers a transparent view of how much of the company’s financial activities contribute to sustainability.
Turnover percentage calculation
The value of the turnover percentage, as set out in Article 8(2)(a) of EU Regulation 2020/852, was calculated by taking the portion of the net turnover attributable to products or services, including intangible assets. This portion is associated with economic activities that comply with the taxonomy and represent the numerator in the calculation, divided by the net turnover (denominator) as defined in Article 2(5) of Directive 2013/34/ EU.
Turnover includes revenue recognized in accordance with International Accounting Standard (IAS) 1, paragraph 82, letter a), adopted by Commission Regulation (EC) No. 1126/2008. In the specific case of Grifols, the numerator in the turnover KPI is calculated as the total turnover (recorded under Group 70 of the General Chart of Accounts) selected based on accounts considered eligible from a taxonomic standpoint. With regard to the numerator of the turnover KPI, Grifols identified "Activity 1.2: Manufacture of pharmaceuticals" as eligible. This corresponds to environmental objective “Pollution Prevention and Control.” Consequently, revenues from the Biopharma functional area have been included in the numerator calculation.
The denominator corresponds to Grifols’ total turnover.
CapEx percentage calculation
The CapEx ratio, as stipulated in Article 8(2)(b) of Regulation (EU) 2020/852, was calculated as the numerator divided by the denominator, the denominator being the additions to tangible and intangible assets during the reporting period before depreciation, amortization and possible revaluations, including those resulting from revaluations and impairments during the relevant period, excluding changes in fair value. The denominator also includes additions to tangible and intangible assets resulting from business combinations.
Specifically, the denominator is Grifols’ total CapEx, which includes investments in intangible assets, investments in property, plant and equipment, investments in assets for rights of use and assets transferred without consideration. The numerator consists solely of the aggregation of the CapEx of the activities considered eligible from a taxonomic standpoint.
OpEx percentage calculation
The OpEx ratio, according to Article 8(2)(b) of Regulation (EU) 2020/852, was calculated by dividing the numerator by the denominator. The OpEx denominator includes non-capitalized direct costs related to research and development, building renovation measures, short-term leases, maintenance and repairs, as well as other direct costs related to the day-to-day maintenance of property, plant and equipment by the company or a third-party subcontractor to ensure the continued efficient operation of these assets. Leasing costs for non-financial companies that apply national GAAP and do not capitalize right-of-use assets are also included in the OpEx.
In this case, the OpEx indicator calculation covers:
- Direct non-capitalized costs associated with research and development
- Short-term leases
- Maintenance and repair costs
However, other expenses related to the day-to-day maintenance of property, plant and equipment, such as cleaning services or repairs to computer systems, have not been included in the numerator calculation, in compliance with Article 8 of the regulations and the accounting methodology adopted by Grifols to present these expenses. Furthermore, as a precautionary measure, those situations in which an expense account was not sufficiently detailed to be able to determine whether it was a maintenance expense directly linked to any of the taxonomic activities analyzed, or other types of maintenance such as those mentioned above, have not been considered as eligible.
Accordingly, the denominator of the indicator includes the total expenditure of these three Grifols items, while the numerator is composed of the same items, but only for the activities that have been recognized as eligible in accordance with the established criteria.
Results
The following tables set out the data corresponding to turnover, CapEx and OpEx for Grifols’ economic activities that comply with the European Taxonomy.
Grifols Results
Turnover
| Financial year 2023 | Year 2023 | Substantial Contribution Criteria | DNSH criteria (‘Does Not Significantly Harm’) | ||||||||||||||||
| Economic Activities (1) | Code (2) | Turnover (3) | Proportion of Turnover 2023 (4) | Climate Change Mitigation (5) | Climate Change Adaption (6) | Water (7) | Pollution (8) | Circular Economy (9) | Biodiversity (10) | Climate Change Mitigation (11) | Climate Change Adaption (12) | Water (13) | Pollution (14) | Circular Economy (15) | Biodiversity (16) | Minimum Safeguards (17)) | Proportion of Taxonomy-aligned (A.1) or eligible (A.2) turnover, 2022 (18)) | Category enabling activity (19)) | Category transitional activity (20) |
| Text | EUR | % | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S/N | S/N | S/N | S/N | S/N | S/N | S/N | % | F | T | |
| A. TAXONOMY-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| A.1. Environmentally sustainable activities (Taxonomy-aligned) | |||||||||||||||||||
| Turnover of environmentally sustainable activities (Taxonomy-aligned) (A.1) | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0% | |||
| Of which Enabling | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0% | F | ||
| Of which Transitional | 0 | 0 | - | - | - | - | - | - | - | - | 0% | T | |||||||
| A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) | |||||||||||||||||||
| 1.2 Manufacture of medicinal products | PPC 1.2 | 5,096,622,614.77 | 83.14% | N/EL | N/EL | N/EL | EL | N/EL | N/EL | *2 | |||||||||
| Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) | 5,096,622,614.77 | 83.14% | 0% | 0% | 0% | 83,14% | 0% | 0% | 0% | ||||||||||
| A. Turnover of Taxonomy eligible activities (A.1+A.2) | 5,096,622,614.77 | 83.14% | 0% | 0% | 0% | 83,14% | 0% | 0% | 0% | ||||||||||
| B. TAXONOMY-NON-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| Turnover of Taxonomynon-eligible activities | 1,033,676,332.41 | 16.86% | |||||||||||||||||
| TOTAL | 6,130,298,947.18 | 100% | |||||||||||||||||
*1 All activities added in 2023 were not checked for taxonomy conformity
*2 Economic activity is to be reported for the first time for FY 2023 or did not exist in the previous year, so no previous year’s figures are reported here.
*3 Not applicable, as alignment is to be reviewed for the first time for the 2024 financial year for the other 4 environmental targets.
| Proportion of turnover/Total turnover | ||
| Taxonomy-aligned per objective | Taxonomy-eligible per objective | |
| CCM | 0% | 0% |
| CCA | 0% | 0% |
| WTR | -*3 | 0% |
| CE | -*3 | 0% |
| PPC | -*3 | 83.14% |
| BIO | -*3 | 0% |
CapEx
| Financial year 2023 | Year 2023 | Substantial Contribution Criteria | DNSH criteria ("Does Not Significantly Harm") | ||||||||||||||||
| Economic Activities (1) | Code (2) | CapEx (3) | Proportion of CapEx 2023 (4) | Climate Change Mitigation (5) | Climate Change Adaption (6) | Water (7) | Pollution (8) | Circular Economy (9) | Biodiversity (10) | Climate Change Mitigation (11) | Climate Change Adaption (12) | Water (13) | Pollution (14) | Circular Economy (15) | Biodiversity (16) | Minimum Safeguards (17)) | Proportion of Taxonomy-aligned (A.1) or eligible (A.2) CapEx, 2022 (18)) | Category enabling activity (19)) | Category transitional activity (20) |
| Text | EUR | % | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S/N | S/N | S/N | S/N | S/N | S/N | S/N | % | F | T | |
| A. TAXONOMY-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| A.1. Environmentally sustainable activities (Taxonomy-aligned) | |||||||||||||||||||
| 7.4 Installation, maintenance and repair of charging stations for electric vehicles in buildings (and parking spaces attached to buildings) | CCM 7.4 | 12,760.39 | 0.008% | S | N/EL | N/EL | N/EL | N/EL | N/EL | S | S | S | S | S | S | S | 0% | ||
| CapEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) | 12,760.39 | 0.008% | 0.008% | 0% | 0% | 0% | 0% | 0% | S | S | S | S | S | S | S | 0% | |||
| Of which Enabling | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0% | F | ||
| Of which Transitional | 0 | 0 | - | - | - | - | - | - | - | - | 0% | T | |||||||
| A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) | |||||||||||||||||||
| 7,3, Instalación. mantenimiento y reparación de equipos de eficiencia energética | CCM 7.3 | 70,435.54 | 0.044% | EL | N/EL | N/EL | N/EL | N/EL | N/EL | *2 | |||||||||
| 1.2. Manufacture of medicinal products | PPC 1.2 | 131,999,777.39 | 82.37% | N/EL | N/EL | N/EL | EL | N/EL | N/EL | *2 | |||||||||
| CapEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) | 132,070,212.93 | 82.417% | 0.044% | 0% | 0% | 82.37% | 0% | 0% | 0% | ||||||||||
| A. CapEx of Taxonomy eligible activities (A.1+A.2) | 132,082,973.32 | 82.42% | 0.052% | 0% | 0% | 82.37% | 0% | 0% | |||||||||||
| B. TAXONOMY-NON-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| CapEx of Taxonomynon-eligible activities | 28,164,150.29 | 17.58% | |||||||||||||||||
| TOTAL | 160,247,123.61 | 100% | |||||||||||||||||
*1 All activities added in 2023 were not checked for taxonomy conformity
*2 Economic activity is to be reported for the first time for FY 2023 or did not exist in the previous year, so no previous year’s figures are reported here.
*3 Not applicable, as alignment is to be reviewed for the first time for the 2024 financial year for the other 4 environmental targets.
| Proportion of CapEx/Total CapEx | ||
| Taxonomy-aligned per objective | Taxonomy-eligible per objective | |
| CCM | 0.008% | 0.052% |
| CCA | 0% | 0% |
| WTR | -*3 | 0% |
| CE | -*3 | 0% |
| PPC | -*3 | 82.37% |
| BIO | -*3 | 0% |
OpEx
| Financial year 2023 | Year 2023 | Substantial Contribution Criteria | DNSH criteria ("Does Not Significantly Harm") | ||||||||||||||||
| Economic Activities (1) | Code (2) | OpEx (3) | Proportion of OpEx 2023 (4) | Climate Change Mitigation (5) | Climate Change Adaption (6) | Water (7) | Pollution (8) | Circular Economy (9) | Biodiversity (10) | Climate Change Mitigation (11) | Climate Change Adaption (12) | Water (13) | Pollution (14) | Circular Economy (15) | Biodiversity (16) | Minimum Safeguards (17)) | Proportion of Taxonomy-aligned (A.1) or eligible (A.2) OpEx, 2022 (18)) | Category enabling activity (19)) | Category transitional activity (20) |
| Text | EUR | % | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S/N | S/N | S/N | S/N | S/N | S/N | S/N | % | F | T | |
| A. TAXONOMY-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| A.1. Environmentally sustainable activities (Taxonomy-aligned) | |||||||||||||||||||
| OpEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0% | |||
| Of which Enabling | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0% | F | ||
| Of which Transitional | 0 | 0 | - | - | - | - | - | - | - | - | 0% | T | |||||||
| A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) | |||||||||||||||||||
| 1.1 Conservation, including restoration, of habitats, ecosystems and species | BIO 1.1 | 123,985.32 | 0.07% | N/EL | N/EL | N/EL | N/EL | N/EL | EL | *2 | |||||||||
| 1.2 Manufacture of medicinal products | PPC 1.2 | 45,176,289.89 | 24.76% | N/EL | N/EL | N/EL | EL | N/EL | N/EL | *2 | |||||||||
| OpEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) | 45,300,275.21 | 24.83% | 0% | 0% | 0% | 24.76% | 0% | 0.07% | 0% | ||||||||||
| A. OpEx of Taxonomy eligible activities (A.1+A.2)*1 | 45,300,275.21 | 24.83% | 0% | 0% | 0% | 24.76% | 0% | 0.07% | |||||||||||
| B. TAXONOMY-NON-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| OpEx of Taxonomynon-eligible activities | 137,135,261.76 | 75.17% | |||||||||||||||||
| TOTAL | 182,435,536.97 | 100% | |||||||||||||||||
*1 All activities added in 2023 were not checked for taxonomy conformity.
*2 Economic activity is to be reported for the first time for FY 2023 or did not exist in the previous year, so no previous year’s figures are reported here.
*3 Not applicable, as alignment is to be reviewed for the first time for the 2024 financial year for the other 4 environmental targets.
| Proportion of OpEx/Total OpEx | ||
| Taxonomy-aligned per objective | Taxonomy-eligible per objective | |
| CCM | 0% | 0% |
| CCA | 0% | 0% |
| WTR | -*3 | 0% |
| CE | -*3 | 0% |
| PPC | -*3 | 24,76% |
| BIO | -*3 | 0,07% |
Scope of the European Taxonomy Analysis
In line with the rest of the Annual Integrated and Sustainability Report, the data tables for Grifols Consolidated and the Biotest Group is presented separately to facilitate data comparison. The taxonomy study for Grifols Consolidated is presented in the previous sections. The eligibility and alignment analysis is published by Biotest Group annually along with management approaches and main application policies at www.biotest.com. The main results are presented in section "Biotest Results" in this Appendix.
Biotest results
Turnover
| Financial year 2023 | Year 2023 | Substantial Contribution Criteria | DNSH criteria (‘Does Not Significantly Harm’) | ||||||||||||||||
| Economic Activities (1) | Code (2) | Turnover (3) | Proportion of Turnover 2023 (4) | Climate Change Mitigation (5) | Climate Change Adaption (6) | Water (7) | Pollution (8) | Circular Economy (9) | Biodiversity (10) | Climate Change Mitigation (11) | Climate Change Adaption (12) | Water (13) | Pollution (14) | Circular Economy (15) | Biodiversity (16) | Minimum Safeguards (17)) | Proportion of Taxonomy-aligned (A.1) or eligible (A.2) turnover, 2022 (18)) | Category enabling activity (19)) | Category transitional activity (20) |
| Text | EUR | % | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S/N | S/N | S/N | S/N | S/N | S/N | S/N | % | F | T | |
| A. TAXONOMY-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| A.1. Environmentally sustainable activities (Taxonomy-aligned) | |||||||||||||||||||
| Turnover of environmentally sustainable activities (Taxonomy-aligned) (A.1) | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | |||
| Of wich Enabling | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | F | ||
| Of wich Transitional | 0 | 0 | - | - | - | - | - | - | - | - | 0 | T | |||||||
| A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) | |||||||||||||||||||
| 1.2 Manufacture of medicinal products | PPC 1.2 | 487,986,519.87 | 71.28 | N/EL | N/EL | N/EL | EL | N/EL | N/EL | - | |||||||||
| Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) | 487,986,519.87 | 71.28 | 0% | 0% | 0% | 71.28% | 0% | 0% | 0% | ||||||||||
| A. Turnover of Taxonomy eligible activities (A.1+A.2) | 487,986,519.87 | 71.28 | 0% | 0% | 0% | 71.28% | 0% | 0% | 0% | ||||||||||
| B. TAXONOMY-NON-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| Turnover of Taxonomynon-eligible activities | 196,612,445.82 | 28.72 | |||||||||||||||||
| TOTAL | 684,598,965.69 | 100% | |||||||||||||||||
*1 All activities added in 2023 were not checked for taxonomy conformity
*2 Economic activity is to be reported for the first time for FY 2023 or did not exist in the previous year, so no previous year’s figures are reported here
*3 Not applicable, as alignment is to be reviewed for the first time for the 2024 financial year for the other 4 environmental targets
| Proportion of turnover/Total turnover | ||
| Taxonomy-aligned per objective | Taxonomy-eligible per objective | |
| CCM | 0% | 0% |
| CCA | 0% | 0% |
| WTR | -*3 | 0% |
| CE | -*3 | 0% |
| PPC | -*3 | 71.28% |
| BIO | -*3 | 0% |
CapEx
| Financial year 2023 | Year 2023 | Substantial Contribution Criteria | DNSH criteria ("Does Not Significantly Harm") | ||||||||||||||||
| Economic Activities (1) | Code (2) | CapEx (3) | Proportion of CapEx 2023 (4) | Climate Change Mitigation (5) | Climate Change Adaption (6) | Water (7) | Pollution (8) | Circular Economy (9) | Biodiversity (10) | Climate Change Mitigation (11) | Climate Change Adaption (12) | Water (13) | Pollution (14) | Circular Economy (15) | Biodiversity (16) | Minimum Safeguards (17)) | Proportion of Taxonomy-aligned (A.1) or eligible (A.2) CapEx, 2022 (18)) | Category enabling activity (19)) | Category transitional activity (20) |
| Text | EUR | % | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S/N | S/N | S/N | S/N | S/N | S/N | S/N | % | F | T | |
| A. TAXONOMY-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| A.1. Environmentally sustainable activities (Taxonomy-aligned) | |||||||||||||||||||
| CapEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | |||
| Of wich Enabling | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | F | ||
| Of wich Transitional | 0 | 0 | - | - | - | - | - | - | - | - | 0 | T | |||||||
| A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (g) | |||||||||||||||||||
| 1.2 Manufacture of medicinal products | PPC 1.2 | 41,522,832.53 | 59.81 | N/EL | N/EL | N/EL | EL | N/EL | N/EL | -*2 | |||||||||
| 3.2 Renovation of existing buildings | CE 3.2 CCM 7.2 CCA 7.2 |
570,854.85 | 0.82 | N/EL | N/EL | N/EL | N/EL | EL | N/EL | -*2 | |||||||||
| 4.1 Electricity generation using solar photovoltaic technology | CCM 4.1 CCA 4.1 |
939,000.00 | 1.35 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0,47 | |||||||||
| 4.25 Producation of heat/cool using waste heat | CCM 4.25 CCA 4.25 |
369,174,23 | 0,53 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | -*2 | |||||||||
| 5.4 Reneal of waste water collection and treatment | CCM 5.4 CCA 5.4 |
189,380.48 | 0.27 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | -*2 | |||||||||
| 6.5 Transport of motorbikes, passenger cars and light commercial vehicles | CCM 6.5 CCA 6.5 |
491,574.41 | 0.71 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 1,26% | |||||||||
| 7.4 Installation, maintenance and repair of charging stations for electric vehicles in buildings (and parking spaces attached to buildings | CCM 7.4 CCA 7.4 |
17,458.00 | 0.03 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0,26% | |||||||||
| 7.5 Installation, maintenance and repair of instruments and devices for measuring, regulation and controlling energy performance of buildings | CCM 7.5 CCA 7.5 | 91,962.00 | 0.13 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0,24% | |||||||||
| 8.1 Data processing, hosting and related activities | CCM 8.1 CCA 8.1 |
260,570.00 | 0.38 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0,03% | |||||||||
| CapEx of Taxonomyeligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) | 44,452,806.50 | 64.03 | 3.40% | 0% | 0% | 59.81% | 0.82% | 0% | 2.35% | ||||||||||
| A. CapEx of Taxonomy eligible activities (A.1+A.2) | 44,452,806.50 | 64.03 | 3.40% | 0% | 0% | 59.81% | 0.82% | 0% | 2.35% | ||||||||||
| B. TAXONOMY-NON-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| Taxonomy-noneligible activities | 24,975,199.89 | 35.97 | |||||||||||||||||
| TOTAL | 69,428,006.39 | 100% | |||||||||||||||||
*1 All activities added in 2023 were not checked for taxonomy conformity
*2 Economic activity is to be reported for the first time for FY 2023 or did not exist in the previous year, so no previous year’s figures are reported here.
*3 Not applicable, as alignment is to be reviewed for the first time for the 2024 financial year for the other 4 environmental targets.
| Proportion of CapEx/Total CapEx | ||
| Taxonomy-aligned per objective | Taxonomy-eligible per objective | |
| CCM | 0% | 4.22% |
| CCA | 0% | 4.22% |
| WTR | -*3 | 0% |
| CE | -*3 | 0.82% |
| PPC | -*3 | 76.02% |
| BIO | -*3 | 0% |
OpEx
| Financial year 2023 | Year 2023 | Substantial Contribution Criteria | DNSH criteria ("Does Not Significantly Harm") | ||||||||||||||||
| Economic Activities (1) | Code (2) | OpEx (3) | Proportion of OpEx 2023 (4) | Climate Change Mitigation (5) | Climate Change Adaption (6) | Water (7) | Pollution (8) | Circular Economy (9) | Biodiversity (10) | Climate Change Mitigation (11) | Climate Change Adaption (12) | Water (13) | Pollution (14) | Circular Economy (15) | Biodiversity (16) | Minimum Safeguards (17)) | Proportion of Taxonomy-aligned (A.1) or eligible (A.2) OpEx, 2022 (18)) | Category enabling activity (19)) | Category transitional activity (20) |
| Text | EUR | % | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S; N; N/EL (b) (c) | S/N | S/N | S/N | S/N | S/N | S/N | S/N | % | F | T | |
| A. TAXONOMY-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| A.1. Environmentally sustainable activities (Taxonomy-aligned) | |||||||||||||||||||
| OpEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | |||
| Of wich Enabling | 0 | 0 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | F | ||
| Of wich Transitional | 0 | 0 | - | - | - | - | - | - | - | - | 0 | T | |||||||
| A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (g) | |||||||||||||||||||
| 1.2 Manufacture of medicinal products | PPC 1.2 | 79,821,353.90 | 89.22 | N/EL | N/EL | N/EL | EL | N/EL | N/EL | -*2 | |||||||||
| 2.4 Remediation of contaminated sites | PPC 2.4 | 35,206.69 | 0.04 | N/EL | N/EL | N/EL | EL | N/EL | N/EL | -*2 | |||||||||
| 4.9 Transmission and distribution of electricity | CCM 4.9 CCA 4.9 |
115,610.00 | 0.13 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.23% | |||||||||
| 4.11 Storage of thermal energy | CCM 4.11 CCA 4.11 |
30,580.00 | 0.03 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.12% | |||||||||
| 4.30 High-efficiency co-generation of heat/cool and power from fossil gaseous fuels | CCM 4.30 CCA 4.30 |
169,943.00 | 0.19 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.16% | |||||||||
| 5.3 Construction, extension and operation of waste water collection and treatment | CCM 5.3 CCA 5.3 |
100,446.00 | 0.11 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.24% | |||||||||
| 5.4 Renewal of waste water collection and treatment | CCM 5.4 CCA 5.4 |
74,085.70 | 0.08 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.41% | |||||||||
| 6.5 Transport by motorbikes, passenger cars and light commercial vehicles | CCM 6.5 CCA 6.5 |
265,677.93 | 0.30 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.33% | |||||||||
| 6.6 Freight transport services by road | CCM 6.6 CCA 6.6 | 91,879.72 | 0.10 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.04% | |||||||||
| 7.3 Installation, maintenance and repair of energy efficiency equipment | CCM 7.3 | 2,523,226.00 | 2.82 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.03 | |||||||||
| 7.5 Installation, maintenance and repair of instruments and devices for measuring, regulation and controlling energy performance of buildings | CCM 7.5 | 255,525.00 | 0.29 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.39% | |||||||||
| 8.1 Data processing, hosting and related activities | CCM 8.1 CCA 8.1 |
471,612.27 | 0.53 | EL | N/EL | N/EL | N/EL | N/EL | N/EL | 0.01% | |||||||||
| OpEx of Taxonomyeligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2)*1 | 83,955,146 | 93.84 | 4.58% | 0% | 0% | 0,00% | 89.26% | 0% | 4.65% | ||||||||||
| A. OpEx of Taxonomy eligible activities (A.1+A.2) | 83,955,146 | 93.84 | 4.58% | 0% | 0% | 0,00% | 89.26% | 0% | 4.65% | ||||||||||
| B. TAXONOMY-NON-ELIGIBLE ACTIVITIES | |||||||||||||||||||
| Taxonomy-noneligible activitie | 5,508,294 | 6.16 | |||||||||||||||||
| TOTAL | 89,463,439.86 | 100% | |||||||||||||||||
*1 All activities added in 2023 were not checked for taxonomy conformity
*2 Economic activity is to be reported for the first time for FY 2023 or did not exist in the previous year, so no previous year’s figures are reported here
*3 Not applicable, as alignment is to be reviewed for the first time for the 2024 financial year for the other 4 environmental targets.
| Proportion of turnover/Total turnover | ||
| Taxonomy-aligned per objective | Taxonomy-eligible per objective | |
| CCM | 0% | 4.58% |
| CCA | 0% | 1.44% |
| WTR | -*3 | 0% |
| CE | -*3 | 0% |
| PPC | -*3 | 89.26% |
| BIO | -*3 | 0% |
Annex VI. Law 11/2018 content index. Information requested by the Law 11/2018
The selected GRI Disclosures below refer to those published in 2016, except those that have undergone and in which case the year of publication is indicated.
| LAW 11/2018 CONTENT INDEX | |||
| Information requested by the Law 11/2018 | Materiality | Page number(s) | Reporting criteria: GRI (last version except indicated) |
| General information | |||
| A brief description of the business model that includes its business environment, its organization and structure | Material | 11, 16-17 | GRI 2-6 (2021) |
| Markets in which it operates | Material | 14-15 | GRI 2-1 (2021) GRI 2-6 (2021) |
| Objectives and strategies of the organization | Material | 19, 28-29, 92, 134, 212, 218 | GRI 2-1 (2021) GRI 2-22 (2021) |
| Main factors and trends that can affect its future evolution | Material | 233-239 | GRI 3-3 (2021) GRI 2-22 (2021) |
| Reporting framework used | Material | 269-270 | GRI 1 (2021) |
| Principle of materiality | Material | 20-27 | GRI 3-1 (2021) GRI 3-2 (2021) |
| Environmental Issues | |||
| Management approach: description and results of the policies related to these issues, as well as the main risks related to those issues related to the group’s activities. | Material | 93-94, 96, 99-100, 221 | GRI 3-3 (2021) |
| Detailed general information | |||
| Detailed information on the actual and predictable effects of the company’s activities on the environment and, when applicable, health and safety | Material | 101-102 | GRI 3-3 (2021) |
| Environmental assessment or certification procedures | Material | 95 | GRI 3-3 (2021) |
| Resources dedicated to the prevention of environmental risks | Material | 97 | GRI 3-3 (2021) |
| Application of the precautionary principle | Material | 93-94, 97 | GRI 2-23 (2021) |
| Amount of provisions and guarantees for environmental risks | Material | 97-98, 119 | GRI 3-3 (2021) |
| Contamination | |||
| Measures to prevent, reduce or repair emissions that seriously affect the environment; considering any form of activity-specific air pollution, including noise and light pol | Material | 104, 120-121 | GRI 3-3 (2021) GRI 305-7 |
| Circular Economy and Waste Prevention and Management | |||
| Prevention, recycling, reutilization and other recovery and waste disposal measures | Material | 110, 114-115, 130-132 | GRI 306-1 GRI 306-2 GRI 306-4 GRI 306-5 |
| Actions to fight food waste | Not material | - | - |
| Sustainable Use of Resources | |||
| Water consumption and supply in accordance with the local limitations | Material | 112-113, 124-128 | GRI 303-1 a 303-5 |
| Consumption of raw materials and measures taken to improve the efficiency of their use | Material | 110-111, 133 | GRI 301-1 |
| Direct and indirect energy consumption | Material | 105-109, 121-124, 129 | GRI 302-1 GRI 302-3 |
| Measures taken to improve energy efficiency | Material | 99, 105, 108 | GRI 3-3 (2021) GRI 201-2 |
| Use of renewable energy | Material | 108, 124 | GRI 302-1 |
| Climate Change | |||
| Greenhouse gas emissions generated as a result of the company’s activities, including the use of the goods and services it produces | Material | 103-104, 119-121 | GRI 305-1 GRI 305-2 GRI 305-3 GRI 305-4 |
| Measures taken to adapt to the consequences of climate change | Material | 102, 104 | GRI 3-3 (2021) GRI 201-2 |
| Voluntary measures for medium and long-term reduction goals to reduce greenhouse gas emissions and the means implemented for this purpose | Material | 102-104 | GRI 3-3 (2021) |
| Biodiversity Protection | |||
| Measures taken to preserve or restore biodiversity | Material | 116-118 | GRI 3-3 (2021) |
| Impacts caused by activities or operations in protected areas | Material | 116-118 | GRI 3-3 (2021) |
| Social and Personnel matters | |||
| Management approach: description and results of the policies related to these matters as well as the main risks related to those issues linked to the group’s activities. | Material | 134-137, 221 | GRI 3-3 (2021) |
| Employment | |||
| Total number and distribution of employees by country, gender, age and professional category | Material | 138-139, 167 - 177 | GRI 405-1 |
| Total number and distribution of employment contract modalities and annual average of indefinite contracts, temporary contracts and part-time contracts by gender, age and professional category | Material | 167-177 | GRI 2-7 (2021) |
| Number of dismissals by gender, age and professional classification | Material | 178-179 | GRI 3-3 (2021) GRI 401-1 |
| Average remuneration and its evolution disaggregated by sex, age and professional classification or equal va | Material | 155, 186-188 | GRI 3-3 (2021) |
| Gender gap, the remuneration of equal or average company jobs | Material | 156-159, 189-190 | GRI 3-3 (2021) GRI 405-2 |
| Average remuneration of directors and executives, including variable remuneration, allowances, allowances, payment to long-term savings forecasting systems and any other perception disaggregated by sex | Material | 31, 188, 219 | GRI 3-3 (2021) |
| Implementation of policies work disconnection | Material | 137, 166 | GRI 3-3 (2021) |
| Number of employees with disabilities | Material | 143 | GRI 3-3 (2021) GRI 405-1 |
| Organization of Work | |||
| Organization of working time | Material | 166 | GRI 3-3 (2021) |
| Number of hours of absenteeism | Material | 180-181 | GRI 3-3 (2021) GRI 403-9 |
| Measures aimed at facilitating the enjoyment of conciliation and promoting the co-responsible exercise of these by both parents | Material | 166 | GRI 3-3 (2021) GRI 403-3 |
| Health and Safety | |||
| Health and safety conditions at work | Material | 162-165 | GRI 3-3 (2021) GRI 403-1 GRI 403-3 GRI 403-4 GRI 403-5 GRI 403-6 GRI 403-7 |
| Occupational accidents, their frequency and severity, as well as occupational diseases; disaggregated by gend | Material | 165, 185 | GRI 403-9 GRI 403-10 |
| Social Relationships | |||
| Organization of social dialogue including procedures for informing and consulting staff and negotiating with them | Material | 160 | GRI 3-3 (2021) |
| Mechanisms and procedures that the company has to promote the involvement of workers in the management of the company, in terms of information, consultation and participation | Material | 160 | GRI 3-3 (2021) |
| Percentage of employees covered by collective agreement by country | Material | 160 | GRI 2-30 (2021) |
| Balance of collective agreements, particularly in the field of health and safety at work | Material | 160 | GRI 3-3 (2021) GRI 403-4 |
| Training | |||
| Policies implemented in the field of training | Material | 136, 150-151 | GRI 404-2 |
| Total number of training hours by professional category | Material | 152, 181-183 | GRI 3-3 (2021) GRI 404-1 |
| Universal accessibility | |||
| Integration and universal accessibility of people with disabilities | Material | 143 | GRI 3-3 (2021) |
| Equality | |||
| Measures taken to promote equal treatment and opportunities for women and men | Material | 136, 140 | GRI 3-3 (2021) |
| Equality plans, measures taken to promote employment, protocols against sexual and gender harassment | Material | 142, 144 - 145 | GRI 3-3 (2021) |
| Policy against all types of discrimination and, when applicable, diversity management | Material | 136, 140-142 | GRI 3-3 (2021) |
| Respect for human rights | |||
| Management approach: description and results of the policies related to these matters as well as the main risks related to those issues linked to the group’s activities. | Material | 32-35, 38, 41, 221 | GRI 3-3 (2021) |
| Application of due diligence procedures | |||
| Application of due diligence procedures in the field of human rights and prevention of risks of violation of human rights and, where appropriate, measures to mitigate, manage and repair possible abuses committed | Material | 32-35 | GRI 2-23 (2021) |
| Complaints for cases of human rights violation | Material | 232 | GRI 3-3 (2021) GRI 406-1 (2016) |
| Measures implemented to promote and comply with the provisions of the ILO fundamental conventions related to respect for freedom of association and the right to collective bargaining; the elimination of discrimination in employment and occupation; the elimination of forced or compulsory labor; the effective abolition of child labor | Material | 32-33, 142 | GRI 3-3 (2021) |
| Fight against corruption and bribery | |||
| Management approach: description and results of the policies related to these matters as well as the main risks related to those issues linked to the group’s activities. | Material | 221, 225-227 | GRI 3-3 (2021) |
| Measures taken to prevent corruption and bribery | Material | 221, 225-231 | GRI 3-3 (2021) GRI 2-23 (2021) GRI 205-1 a 205-3 |
| Measures to fight money laundering | Material | 227 | GRI 3-3 (2021) GRI 2-23 (2021) GRI 205-1 a 205-3 |
| Contributions to foundations an NGOs | Material | 228-229, 278-279 | GRI 2-28 (2021) GRI 201-1 GRI 415-1 |
| Information about society | |||
| Management approach: description and results of the policies related to these matters as well as the main risks related to those issues linked to the group’s activities | Material | 194-197, 221 | GRI 3-3 (2021) |
| Commitment of the company to sustainable development | |||
| The impact of the company’s activity on employment and local development | Material | 197-201 | GRI 3-3 (2021) GRI 203-2 |
| The impact of society’s activity on local populations and in the territory | Material | 194, 196-197, 199 | GRI 3-3 (2021) |
| The relations maintained with the actors of the local communities and the modalities of the dialogue with these | Material | 26-27,195-201 | GRI 2-29 (2021) |
| Partnership or sponsorship actions | Material | 195-201, 208-211, 279 | GRI 3-3 (2021) GRI 201-1 |
| Subcontracting and suppliers | |||
| Inclusion in the purchasing policy of social, gender equality and environmental issues | Material | 37-38, 41 | GRI 3-3 (2021) |
| Consideration in the relations with suppliers and subcontractors of their social and environmental responsibility | Material | 37-39, 41 | GRI 2-6 (2021) |
| Supervision and audit systems and their results | Material | 39, 51 | GRI 2-6 (2021) |
| Consumers | |||
| Measures for the health and safety of consumers | Material | 44, 46-50, 222-224 | GRI 3-3 (2021) GRI 416-1 |
| Complaint systems, complaints received and resolution thereof | Material | 48 | GRI 3-3 (2021) GRI 418-1 |
| Tax information | |||
| Profit obtained country by country | Material | 267 | GRI 3-3 (2021) GRI 207-4 |
| Taxes earned on benefits paid (per country) | Material | 261, 267 | GRI 3-3 (2021) GRI 201-1 GRI 207-4 |
| Public grants received (per country) | Material | 251 | GRI 3-3 (2021) |
| EU Taxonomy | Material | 280-289 | KPIs developed according to the methodology described in this report |
Annex VII. GRI Content Index
| GRI Standards | GRI Disclosure | Page number, URL and/or direct response | Omission | |
| Statement of use | Grifols S.A. has reported in accordance with the GRI Standards for the period January 1st to December 31st of 2023. | |||
| GRI 1: Foundation 2021 | ||||
| General Disclosures | ||||
| GRI 2: General Disclosures 2021 | The organization and its reporting practices | |||
| 2-1 | Organizational details | 14-15,266-267 | ||
| 2-2 | Entities included in the organization’s sustainability reporting | 271-272 | ||
| 2-3 | Reporting period, frequency and contact point | 271 Contact: investors@grifols.com / sustainability@grifols.com |
||
| 2-4 | Restatements of information | All information with a temporal or organitzational scope other than 2022 or 2021 is properly indicated and accompanied by a clarification. | ||
| 2-5 | External assurance | 301-302 | ||
| Activities and workers | ||||
| 2-6 | Activities, value chain, and other business relationships | 11-17, 37-39 | No information available related to the requirement b-iii | |
| 2-7 | Employees | 167-177 Grifols does not hire employees without assigned hours |
||
| 2-8 | Workers who are not employees | Not applicable | ||
| Governance | ||||
| 2-9 | Governance structure and composition | 213-218 IAGC (section C) https://www.grifols.com/en/annual-corporate-governance-report |
||
| 2-10 | Nomination and selection of the highest governance body | IAGC (section C) https://www.grifols.com/en/annual-corporate-governance-report Policy on Director Diversity in the composition of the Board of Directors Grifols S.A https://www.grifols.com/documents/3625622/3684243/Grifols+-+Politica+de+diversidad++-+Dic.+2020+-+ES.PDF/e054c860-a308-46eb-af53-5ca7b187e0dd?t=1608130227711 |
||
| 2-11 | Chair of the highest governance body | 214-217 | ||
| 2-12 | Role of the highest governance body in overseeing the management of impacts | 30 | ||
| 2-13 | Delegation of responsibility for managing impacts | 30 | ||
| 2-14 | Role of the highest governance body in sustainability reporting | 30 | ||
| 2-15 | Conflicts of interes | IAGC (section C) https://www.grifols.com/en/annual-corporate-governance-report |
||
| 2-16 | Communication of critical concerns | 214 | ||
| 2-17 | Collective knowledge of the highest governance body | 153 | ||
| 2-18 | Evaluation of the performance of the highest governance body | IAGC (section C) https://www.grifols.com/en/annual-corporate-governance-report |
||
| 2-19 | Remuneration policies | 31, 219-220 Remuneration Policy for Directors of Grifols, S.A. https://www.grifols.com/documents/3625622/5421064/Directors-Remuneration-Policy-proposal-ES.pdf/6c8473e3-947d-0d5f-1d6b-e3bb992fa8ff?t=1686904260735 |
||
| 2-20 | Process to determine remuneration | Remuneration Policy for Directors of Grifols, S.A. https://www.grifols.com/documents/3625622/5421064/Directors-Remuneration-Policy-proposal-ES.pdf/6c8473e3-947d-0d5f-1d6b-e3bb992fa8ff?t=1686904260735 |
||
| 2-21 | Annual total compensation ratio | Not reported due to confidentiality constraints | ||
| Strategy, policies and practices | ||||
| 2-22 | Statement on sustainable development strategy | 6, 19 | ||
| 2-23 | Policy commitments | 19, 28-29, 38, 92-94, 134, 136, 212, 218, 221 | ||
| 2-24 | Embedding policy commitments | 19, 28-29, 38, 92-94, 134, 136, 212, 218, 221 | ||
| 2-25 | Processes to remediate negative impacts | 46, 226-227, 232 | ||
| 2-26 | Mechanisms for seeking advice and raising concerns | 27, 232 "During the course 2023, Biotest has not received any formal complaints through its designated channel. However, the company have received receive 9 communications through internal reports to higher positions or the Human Resources team. Among these communications, 2 correspond to matters concerning compliance with standards, norms, processes, or laws, as well as inappropiate behavior. No formal reports or complaints have been made regarding sexual harassment, discrimination, conflict of interest, health and safety or corruption" |
||
| 2-27 | Compliance with laws and regulations | 49-50, 97, 222, 225-226, 232 | ||
| 2-28 | Membership associations | 228-229, 278-279 | ||
| Stakeholder engagement | ||||
| 2-29 | Approach to stakegolder engagement | 26-27 | ||
| 2-30 | Collective bargaining agreements | 160 | ||
| Material Topics | ||||
| GRI 3: Material Topics 2021 | 3-1 | Process to determine material topics | 20-25 | |
| 3-2 | List of material topics | 21, 24-25 | ||
| Circular economy and resource management | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 93-94, 99-100, 221 | No information available related to the requirements a, b, d, e, f |
| GRI 301: Materials 2016 | 301-1 | Materials used by weight or volume | 133 | Given the nature of the materials used by Grifols, the breakdown by renewable and non-renewable is not applicable. |
| GRI 303: Water and Effluents 2018 | 303-1 | Interactions with water as a shared resource | 93, 100, 112-113 | No information available related to the requirement c. |
| 303-2 | Management of water discharge-related impacts | 113 | ||
| 303-3 | Water withdrawl | 112-113, 124-128 | No information available related to the requirement c. | |
| 303-4 | Water discharge | 112-113, 124-128 | No information available related to the requirements b, c. | |
| 303-5 | Water consumption | 112-113, 124-128 | ||
| GRI 306: Waste 2020 | 306-1 | Waste generation and significant waste-related impacts | 110, 114-115 | |
| 306-2 | Management of significant waste-related impacts | 110, 114-115 Management platforms, tracking sheets, internal spreadsheets and reports from waste managers are used to collect and track data associated with waste quantities. This data is fed into the SAP Sustainability Performance Management platform. |
Information regarding significant waste-related impacts is not available for publication in this report. Specific measures are being taken in the collection of information and the data processing process to be able to provide this detail in the next five years. | |
| 306-4 | Waste diverted from disposal | 114, 130-131 | No information available related to the requirement d. | |
| 306-5 | Waste directed to disposal | 114, 130-131 | No information available related to the requirement d. | |
| Climate change | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 93-94, 99, 101-104, 221 | No information available related to the requirements a, b, d, e, f |
| GRI 201: Economic Performance 2016 | 201-2 | Financial implications and other risks and opportunities due to climate change | 101-102, 119 | |
| GRI 305: Emissions 2016 | 305-1 | Direct (Scope 1) GHG emissions | 103-104, 119-121 | |
| 305-2 | Energy indirect (Scope 2) GHG emissions | 103-104, 119-121 | ||
| 305-3 | Other indirect (Scope 3) GHG emissions | 103-104, 119-121 | ||
| 305-4 | GHG emissions intensity | 121 | ||
| 305-6 | Emissions of ozone-depleting substances (ODS) | 104 | No information available related to the requirements a, c. | |
| 305-7 | Nitrogen oxides (NOX), sulfur oxides (SOX), and other significant air emissions | 103-104,120-121 | No information available related to the requirements a-iii, iv, v, v. | |
| Energy Efficienc | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 93-94, 99, 105-109, 221 | No information available related to the requirements a, b, d, e, f |
| GRI 302: Energía 2016 | 302-1 | Energy consumption within the organization | 105-109, 121-124 | |
| 302-3 | Energy intensity | 122-124 All ratios are reported using energy consumption within the organization |
||
| 302-4 | Reduction of energy consumption | 105-109 | ||
| Human rights | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 32-35, 38, 41, 221 | No information available related to the requirements a, b, d, e, f |
| Ethical code and good business practices | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 221 | No information available related to the requirements a, b, d, e, f |
| GRI 205: Anti-corruption 2016 | 205-1 | Operations assessed for risks related to corruption | 225, 227, 235-236 | No information available related to the requirement a, regarding to the percentage of operations assessed for risks related to corruption. |
| 205-2 | Communication and training about anti-corruption policies and procedures | Total number of governance body members that the organization’s
anti-corruption policies and procedures have been communicated to: 3
(USA) and 26 (Europe) for Grifols and 6 (Europe) for Biotest. Total: 35. Total number of governance body members that have received training on anti-corruption: Grifols: 3 (USA) and 26 (Europe) and Biotest: 6 (Europe). Total: 35. Grifols’ employees most likely to witness cases of corruption have been informed of anti-corruption policies and procedures in 2023: For Biotest it’s 89 employees (Europe) and 1,029 for Grifols (global). Total: 1,118. The breakdown for Grifols regarding region and employee category is as follows: Administratives/Manufacturing: 13 in Europe, 10 in USA and 9 in the rest of the world. Directors: 31 in Europe, 67 in USA and 10 in the rest of the world. Executives: 9 in Europe, 7 in USA and 1 in the rest of the world. Management: 107 in Europe, 43 in USA and 29 in the rest of the world. Professional: 142 in Europe, 15 in USA and 34 in the rest of the world. Senior management: 80 in Europe, 57 in USA and 19 in the rest of the world. Senior Professional: 87 in Europe, 226 in USA and 33 in the rest of the world. Grifols’ employees most likely to witness cases of corruption have undergone specific training on anticorruption in 2023: For Biotest it’s 85 employees (Europe) and 990 in Grifols (global). Total: 1,075. The breakdown for Grifols regarding region and employee category is as follows: Administratives/Manufacturing: 10 in Europe, 9 in USA and 8 in the rest of the world. Directors: 31 in Europe, 61 in USA and 10 in the rest of the world. Executives: 9 in Europe, 7 en in USA and 1 in the rest of the world. Management: 104 in Europe, 40 in USA and 29 in the rest of the world. Professional: 136 in Europe, 15 in USA and 34 in the rest of the world. Senior management: 80 in Europe, 54 in USA and 19 in the rest of the world. Senior Professional: 85 in Europe, 216 in USA and 32 in the rest of the world. |
No information available related to the requirements c | |
| 205-3 | Confirmed incidents of corruption and actions taken | 226-227, 232 | ||
| GRI 207: Tax 2019 | 207-1 | Approach to ta | 261-264 | |
| 207-2 | Tax governance, control, and risk management | 262-264 | ||
| 207-3 | Stakeholder engagement and management of concerns related to tax | 262-264 | ||
| 207-4 | Country-by-country reporting | 172, 267 | No information available related to
the requirements b-i, b-ii, b-iv, b-v,
b-vii, b-ix, b-x. Breakdown of country-by-country information is not available for publication in this report. |
|
| GRI 415 Public Policy (2016) | 415 -1 | Political contributions | 230 | |
| GRI 417 Marketing and Labeling | 417-3 | Incidents of non-compliance concerning marketing communications | 50 | |
| Health contribution (patients and society | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 63-71, 221 | No information available related to the requirements a, b, d, e, f |
| Employee commitment | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 134-137, 221 | No information available related to the requirements a, b, d, e, f |
| GRI 401: Employment 2016 | 401-1 | New employee hires and employee turnove | New hires by region: United States: 5,168 employees, rate 37.13% Europe: 970 employees, rate 14.52% Rest of the world: 108 employees, rate 19.85% New hires by age group: <30: 3,521 employees, rate 61.75% 30-50: 2,318 employees, rate 21.21% >50: 407 employees, rate 9.02% Total number of departures and staff turnover rate by region: United States: 7,800 employees, rate 56.04% Europe: 997 employees, rate 14.92% Rest of the world: 97 employees, rate 17.83% Total number of departures and staff turnover rate by age group: <30: 3,946 employees, rate 69.2% 30-50: 3,800 employees, rate 34.76% >50: 1,148 employees, rate 25.45% |
|
| 401-2 | Benefits provided to full-time employees that are not provided to temporary or part-time employees | All employees of the main locations with the exception of the US
receive the same benefits and labor benefits according to their category
regardless of the type of contract (full or part time). In the US, all full-time workers who work an average of 30 hours or more a week, as well as their partner and children, have various insurance policies (Life insurance, group accident insurance, short-term work disability insurance). term and long-term and work-related travel accident insurance). They also participate in the Employee Assistance Program, a health and wellness program (LiveWell Wellness Incentive Program and Gympass), 401k Match, reimbursement for training, vacation pay (PTO Pay, Holiday Pay) and have adoption assistance. Part-time workers receive 401k, work-related travel accident insurance, participate in the Employee Assistance Program and the LiveWell Wellness Incentive Program and Gympass. |
||
| 401-3 | Parental leave | 184 | ||
| GRI 402: Labor/management relations | 402-1 | Minimum notice periods regarding operational changes | Significant operational changes in the organization that may substantially affect employees are notified with the minimum notice established in compliance with applicable legislation and collective bargaining agreements. | |
| GRI 405: Diversity and Equal Opportunity 2016 | 405-1 | Diversity of governance bodies and employees | 215-216, 172-177 | |
| 405-2 | Ratio of basic salary and remuneration of women to men | 189-190 | ||
| GRI 406: Non-discrimination 2016 | 406-1 | Incidents of discrimination and corrective actions taken | 142 | |
| GRI 403: Occupational Health and Safety 2018 | 403-1 | Occupational health and safety management system | 162-163, 165 | |
| 403-3 | Occupational health services | 162-163, 165 | ||
| 403-4 | Worker participation, consultation, and communication on occupational health and safety | 160 | ||
| 403-5 | Worker training on occupational health and safety | 162-163 | ||
| 403-6 | Promotion of worker health | 162-164 | ||
| 403-7 | Prevention and mitigation of occupational health and safety impacts directly linked by business relationships | 162-163 | ||
| 403-9 | Work-related injuries | 165, 185 | No information available related to the requirements b-i, b-ii, b-iv, b-v. | |
| 403-10 | Occupational diseases | 165, 185 | No information available related to the requirements b. | |
| Data protection and cybersecurity | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 221-224 | No information available related to the requirements a, b, d, e, f |
| GRI 418: Customer Privacy 2016 | 418-1 | Substantiated complaints concerning breaches of customer privacy and losses of customer data | 222 | |
| Innovation and knowledge generation | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 73, 75-77, 80-81, 83, 86, 89, 150-151, 221 | No information available related to the requirements a, b, d, e, f |
| GRI 404: Training and education 2016 | 404-1 | Average hours of training per year per employee | Average hours of training per employee by gender: Women 347.54
hours and Men 210.04 hours. By professional category: Executives: 16,2h Directors: 33,73h Senior Management: 42,41h Management: 53,15h Senior Professional: 58.83h Professional: 133,17h Administrative/Manufacturing operators: 401.47h Average hours of training per employee calculated from the cumulative average workforce for the year (FTE average) |
|
| 404-2 | Programs for upgrading employee skills and transition assistance programs | 148-149,153 | ||
| 404-3 | Percentage of employees receiving regular performance and career development reviews | 184 | ||
| Contribution to society | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 194-197,221 | No information available related to the requirements a, b, d, e, f |
| GRI 203: Indirect Economic Impacts 2016 | 203-1 | Infrastructure investments and services supported | 195-201 | |
| Product safety and quality | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 46-50, 221 | No information available related to the requirements a, b, d, e, f |
| GRI 416: Customer Health and Safety 2016 | 416-1 | Assessment of the health and safety impacts of product and service categories | 46-51 | |
| 416-2 | Incidents of non-compliance concerning the health and safety impacts of products and services | 47-50 | ||
| Plasma and donors | ||||
| GRI 3: Material Topics 2021 | 3-3 | Management of material topics | 53, 55, 60, 221 Coverage: Within and outside of the organization. The organization contributes directly to the impact. |
No information available related to the requirements a, b, d, e, f |
Annex VIII. SASB Content Index
| Sustainability Accounting Standards Board (SASB) - Biotechnology & Pharmaceuticals | ||
| FY23 | ||
| SASB Indicator | Accounting metric | Disclosure and/or references |
| Safety of Clinical Trial Participants | ||
| HC-BP-210a.1 | Discussion, by world region, of management process for ensuring quality and patient safety during clinical trials |
76-77, 224 For more information please visit https://www.clinicaltrialsregister.eu/ctr-search/search/ https://www.clinicaltrials.gov/ https://eudract.ema.europa.eu/ |
| HC-BP-210a.2 | Number of FDA Sponsor Inspections related to clinical trial management and pharmacovigilance that resulted in: (1) Voluntary Action Indicated (VAI) and (2) Official Action Indicated (OAI) | Grifols has not received any FDA Sponsor Inspections related to clinical trial management and pharmacovigilance that resulted in VAI or OAI. |
| HC-BP-210a.3 | Total amount of monetary losses as a result of legal proceedings associated with clinical trials in developing countries | There has not been any monetary loss as a result of legal proceedings associated with clinical trials in developing countries |
| Access to Medicines | ||
| HC-BP-240a.1 | Description of actions and initiatives to promote access to health care products for priority diseases and in priority countries as defined by the Access to Medicine Index | 65-67 |
| HC-BP-240a.2 | List of products on the WHO List of Prequalified Medicinal Products as part of its Prequalification of Medicines Programme (PQP) | Grifols has no products on the WHO List of Prequalified Medicinal Products |
| Affordability & Pricing | ||
| HC-BP-240b.1 | Number of settlements of Abbreviated New Drug Application (ANDA) litigation that involved payments and/or provisions to delay bringing an authorized generic product to market for a defined time period | Grifols does not market generic products |
| HC-BP-240b.2 | Percentage change in: (1) average list price and (2) average net price across U.S. product portfolio compared to previous year | This information is not reported regarding confidentiality issues |
| HC-BP-240b.3 | Percentage change in: (1) list price and (2) net price of product with largest increase compared to previous year | This information is not reported regarding confidentiality issues |
| Drug Safety | ||
| HC-BP-250a.1 | List of products listed in the Food and Drug Administration’s (FDA) MedWatch Safety Alerts for Human Medical Products database | Information available on the FDA Safety Information and Adverse Event Reporting Program website: |
| HC-BP-250a.2 | Number of fatalities associated with products as reported in the FDA Adverse Event Reporting System | Information available on the FDA Adverse Event Reporting System (FAERS) Public Dashboar: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard |
| HC-BP-250a.3 | Number of recalls issued, total units recalled. | 47 |
| HC-BP-250a.4 | Total amount of product accepted for takeback, reuse, or disposal | We do not accept the return of products for reuse. We collect the products for disposal in accordance with the legal requirements of each country. |
| HC-BP-250a.5 | Number of FDA enforcement actions taken in response to violations of current Good Manufacturing Practices (cGMP), by type | Grifols has not received any FDA enforcement action associated with warning letters, seizures, recalls or consent decrees in 2021. |
| Counterfeit Drugs | ||
| HC-BP-260a.1 | Description of methods and technologies used to maintain traceability of products throughout the supply chain and prevent counterfeiting. | 49 |
| HC-BP-260a.2 | Discussion of process for alerting customers and business partners of potential or known risks associated with counterfeit products. | 49 |
| HC-BP-260a.3 | Number of actions that led to raids, seizure, arrests, and/or filing of criminal charges related to counterfeit products. | 49 |
| Ethical Marketing | ||
| HC-BP-270a.1 | Total amount of monetary losses as a result of legal proceedings associated with false marketing claims | 50 |
| HC-BP-270a.2 | Description of code of ethics governing promotion of off-label use of products | 50 |
| Employee Recruitment, Development & Retention | ||
| HC-BP-330a.1 | Discussion of talent recruitment and retention efforts for scientists and research and development personnel | 148-149 |
| HC-BP-330a.2 | (1) Voluntary and (2) involuntary turnover rate for: (a) executives/senior managers, (b) midlevel managers, (c) professionals, and (d) all others | 178 |
| upply Chain Management | ||
| HC-BP-430a.1 | Percentage of (1) entity’s facilities and (2) Tier I suppliers’ facilities participating in the Rx-360 International Pharmaceutical Supply Chain Consortium audit program or equivalent third party audit programs for integrity of supply chain and ingredients | Grifols does not have facilities that participate in the Rx-360 International Pharmaceutical Supply Chain Con sortium audit program or equivalent programs. However, our facilities are frequently audited by the respective Health authorities of the countries in which we distribute our products. Our suppliers are audited by our own teams of auditors that ensure compliance with all the requirements requested by the health authorities. |
| Business Ethics | ||
| HC-BP-510a.1 | Total amount of monetary losses as a result of legal proceedings associated with corruption and bribery | 226 |
| HC-BP-510a.2 | Description of code of ethics governing interactions with health care professionals | 228 - 229 |
| Activity metrics | ||
| HC-BP-000.A | Number of patients treated | 53, 64 |
| HC-BP-000.B | Number of drugs (1) in portfolio and (2) in research and development (Phases 1-3) | 12-13, 78-80, 82-84 portfolio available at www.grifols.com |
Annex IX. Index of the SDGs and principles of the United Nations Global Compact to which Grifols contributes
This index collects the main SDGs and principles of the United Nations Global Compact to which Grifols contributes with its activity. The main areas of Grifols’ contributions include references to indicate where additional information can be found in the 2023 Integrated Annual Report.
| SDG | Targets | Block within the Integrated Annual Report | Chapter within the Integrated Annual Report | Section within the Integrated Annual Report | Detailed information on the contribution | Related United Nations Global Compact Principles | |
| Priority objectives |  SDG 3 Good health and well-being |
3.3. End the epidemics of AIDS,
tuberculosis, malaria, and neglected
tropical diseases and combat
hepatitis, water-borne diseases, and
other communicable diseases 3.4. Reduce pre-mature mortality from non-communicable diseases (NCDs) by one-third through prevention and treatment and promote mental health and wellbeing. |
1. Understanding Grif | ||||
| Sustainability and Human rights, p. 18-35 |
|
||||||
| Grifols’ value chain, p. 36-51 |
|
||||||
| Donors and patients, p. 52-71 | |||||||
| Innovation at Grifols | Treatment innovations | Maximizing Biotest’s full potential, p. 79 We promote wide-ranging in-house initiatives, p. 80 Milestones and advances in plasma therapies, p. 81 Other initiatives in neurodegenerative diseases, p. 82 GigaGen, non-plasma innovations, p. 83 |
|||||
| Innovation in Diagnostics | Milestones and product launches, p. 84-85 | ||||||
| 2. ESG | Social: Community investment and social outreach | Initiatives through foundations and NGO | Probitas Foundation: improving the health of the most vulnerable populations, p. 203-205 | ||||
 SDG 8 Decent work and economic growth |
8.5. Provide decent work for all women and men, including young people and persons with disabilities through full and productive employment with equal p | 2. ESG | Social: Grifols’ greatest asset | We grow alongside our team | We grow alongside our team, p. 138 Diversity and inclusion, p. 140 Anti-discrimination principles and actions, p. 142 Integrating people with disabilities, p. 143 Equal opportunity plans, p. 144 A holistic understanding of equality, p. 144 Women in Grifols, p. 145 |
|
|
| Fair compensation practices | Remuneration system, p. 155 Moving towards pay equity, p. 156-1577 Grifols continues to make progress towards parity, p. 158-159 |
|
|||||
| 3. Sustainable growth | Grifols’ value creation, p. 257-260 | ||||||
| 8.8. Protect labor rights and promote safe and secure working environments for all workers. | 2. ESG | Social: Grifols’ greatest asset | Occupational health and well-being | Mental Health Policy, leading by example, p. 162 Integrated health and safety management, p. 163 Promoting our employees’ health and well-being, p. 164 Performance in occupational health and safety, p. 165 Absenteeism, p.165 Work-life balance, p. 166 |
|
||
 SDG 9 Industry, innovation and infrastructure |
9.4. Upgrade infrastructure and retrofit industries to make them sustainable and with increased resources use efficiency and greater adoption of clean and environmentally sound technologies and industrial processes. | 1. Understanding Grifols | Donors and patient | Access to treatment and diagnosis | Program to promote countries’ self-sufficiency in plasma and plasma-derived medicines: leading the change, p. 65-67 | ||
| Innovation at Grifols | A robust innovation system, p. 73 | ||||||
| New leadership, p. 74 | |||||||
| Digital innovation, p. 86-87 | |||||||
| 9.5 Enhance scientific research, upgrade the technological capabilities of industrial sectors in all countries, including encouraging innovation and substantially increasing the number of research and development workers and public and private research and development spending. | 1. Understanding Grifols | Innovation at Grifols | Resources allocated to R&D+i, p. 75 | ||||
| Ethics science and innovation | Our commitments, p. 77 | ||||||
| Research collaborations and support | Sponsorship of ISR Program, p. 89 Grifols Chair for the study of Chirrosis and Albumin, p. 89 Grifols Scientific Awards and research grants, p. 89 Scientific journal specialized in plasma, p. 89 |
||||||
 SDG 12 Responsible consumption and production |
12.2. Achieve sustainable management and efficient use of natural resources. | 2. ESG | Environmental | Environmental management at Grif | A cross-cutting and comprehensive approach, p. 93 |
|
|
| A continually evolving internal regulatory | Environmental certifications, p. 94-95 | ||||||
| Environmental governance and climate change action, p. 96 | |||||||
| A global organization to manage environmental risks, p. 97 | |||||||
| Resources allocated to environmental management and climate change, p. 98 | |||||||
| 2023-2026 Environmental Program, p. 99-100 | |||||||
| Energy sources: responsible consum | Natural gas, p.106 Electricity, p. 107 Renewable energies, p. 108 |
||||||
| Circular economy | Consumption of raw materials, p. 111 | ||||||
| Water cycle, p. 112-113 | |||||||
| 12.5. Substantially reduce waste generation through prevention, reduction, recycling, and reuse. | 2. ESG | Environmental | Circular economy, p. 110-111 | ||||
| Waste, p. 114-115 | |||||||
 SDG 13 Climate action |
13.1. Strengthen resilience and adaptive capacity to climate-related hazards and natural disasters in all countries | 1. Understanding Grif | Sustainability and Human rights | Sustainability as a roadmap | Materiality, p. 20-25 |
|
|
| Objectives with a clear timeline: Grifols 2030 Agenda, p. 28-29 | |||||||
| 2. ESG | Environmental | Climate action | The impact of climate change on Grifols, p. 101-102 Emissions, p. 103-104 |
||||
| Relevant objectives |  SDG 4 Quality education |
4.3. Ensure equal access for all women and men to affordable and quality technical, vocational, and tertiary education. | 2. ESG | Social: Grifols’ greatest asset | Talent development | Talent development, p.146 Grifols Performance System, p. 147 People development programs, p. 148 Attracting new talent, p. 149 Student internships, p. 149 |
|
| Driving continuous development | Our culture of learning in 2023, p. 150 Employee development innovations, p. 151 Overview of Grifols employee development, p. 152 |
||||||
| 4.5. Eliminate gender disparities in education by ensuring equal access to all levels of educational and vocational training for the vulnerable, including persons with disabilities, indigenous peoples, and children in vulnerable situations | 2. ESG | Social: Community investment and social outreach | Social action | Health and well-being, p. 198 Education, p. 200 |
|||
| Initiatives through foundations and NGO | Probitas Foundation: improving the health of the most vulnerable populations, p. 203-205 Víctor Grífols Lucas Foundation: bioethics as a principle, p. 206-208 José Antonio Grífols Lucas Foundation: supporting donor communities, p. 209-211 |
||||||
 SDG 5 Gender equality |
5.1. End all forms of discrimination against women and girls everywhere. 5.5. Ensure equal opportunities for leadership and full and effective participation for women at all levels of decision-making in political, economic, and public lif |
2. ESG | Social: Grifols’ greatest asset |
We grow alongside our team | Diversity and inclusion, p. 140-141 Anti-discrimination principles and actions, p. 142 Equal opportunity plans, p. 144 A holistic understanding of equality, p. 144 Women in Grifols, p. 145 |
|
|
| Fair compensation practices | Remuneration system, p. 155 Moving towards pay equity, p. 156-157 Grifols continues to make progress towards parity, p. 158-159 |
|
|||||
| Social: Community investment and social outreach | Social action | Sponsorships: Supporting women in sport, p. 202 | |||||
 ODS 10 Reduced inequalities |
10.2. Empower and promote the social, economic and political inclusion of all irrespective of age, sex, disability, race, ethnicity, origin, religion or economic or other status. | 1. Understanding Grifo | Donors and patients | Serving as a bridge between donors and patients, p. 53 | |||
| Donation centers in committed communities, p. 62 | |||||||
| Access to treatment and diagnosis, p. 65-6 | |||||||
| Patient associations, p. 69-71 | |||||||
| 2. ESG | Social: Helping create more sustainable health systems | The value of our collaboration, p. 191 | |||||
| Optimizing health costs, p. 192 | |||||||
| Collaborations with blood banks, p. 193 | |||||||
| Social: Community investment and social outreach | Principles, our stakeholder groups and scope, p. 194 | ||||||
| Total contributions in 2023, p. 195 | |||||||
| Social action, p. 196-201 | |||||||
| Initiatives through foundations and NGOs | Probitas Foundation: improving the health of the most vulnerable populations, p. 203-205 Víctor Grífols Lucas Foundation: bioethics as a principle, p. 206-208 José Antonio Grífols Lucas Foundation: supporting donor communities, p. 209-211 |
||||||
 SDG 16 Peace, justice and strong institutions |
16.5 Substantially reduce corruption and bribery in all its forms. | 3. ESG | Governance | We promote integrity | Integrated anti-corruption model, p. 226-227 |
|
|
| Grifols Ethics Line, p. 232 | |||||||
| 16.10 Ensure public access to information and protect fundamental freedoms, in accordance with national legislation and international agreements. | 1. Understanding Gri | Sustainability and Human rights | Human rights: an essential pillar, p. 32-35 | ||||
| 2. ESG | Governance | We are transparent, p. 228-231 |
|
||||
| Cross-cutting objectives |  SDG 17 Partnerships for the goals |
17.6 Enhance North-South, SouthSouth and triangular regional and international cooperation on and access to science, technology and innovation, and enhance knowledge sharing on mutually agreed terms, including through improved coordination among existing mechanisms, particularly at UN level, and through a global technology facilitation mechanism when agreed. | 1. Understanding G | Donors and patients | Access to treatment and diagnosis | Program to promote countries’ self-sufficiency in plasma and plasma-derived medicines: leading the change, p. 65-67 | |
| 2. ESG | Social: Helping create more sustainable health systems | The value of our collaboration, p. 191 | |||||
| Optimizing health costs, p. 192 | |||||||
| Collaborations with blood banks, p. 193 | |||||||
| Social: Community investment and social outreach | Initiatives through foundations and NGOs | Probitas Foundation: improving the health of the most vulnerable populations, p. 203-205 Víctor Grífols Lucas Foundation: bioethics as a principle, p. 206-208 José Antonio Grífols Lucas Foundation: supporting donor communities, p. 209-211 |
|||||
| 17.16 Enhance the global partnership for sustainable development, complemented by multi-stakeholder partnerships that mobilize and share knowledge, expertise, technology and financial resources, to support the achievement of the sustainable development goals in all countries, in particular developing countries. | 1. Understanding Grifo | Innovation at Grifols | Treatment innovations | Maximizing Biotest’s full potential, p. 79 We promote wide-ranging in-house initiatives, p. 80 Milestones and advances in plasma therapies, p. 81 Other initiatives in neurodegenerative diseases, p. 82 GigaGen, non-plasma innovations, p. 83 |
|||
| Digital innovation, p. 86-87 | |||||||
| Research collaborations and support | Sponsorship of ISR Program, p. 89 Grifols Chair for the study of Chirrosis and Albumin, p. 89 Grifols Scientific Awards and research grants, p. 89 Scientific journal specialized in plasma, p. 89 |
||||||
| 2. ESG | Social: Grifols’ greatest asset | Driving continuous development | Overview of Grifols employee development 2023, p. 152 Training programs, p. 153 Grifols Academy: differential learning opportunities, p. 154 |
||||
| 17.17 Encourage and promote effective public, public-private and civil society partnerships, building on the experience and resourcing strategies of partnerships. | 2. ESG | Environmental | Waste | Medicine waste management, p. 115 | |||
| Biodiversity, p. 116-11 | |||||||
| Social: Helping create more sustainable health systems | The value of our collaboration, p. 191 | ||||||
| Optimizing health costs, p. 192 | |||||||
| Collaborations with blood banks, p. 193 | |||||||
| Social: Community investment and social outreach | Social action | Environmental, p. 201 | |||||
Annex X. NON-GAAP measures reconciliation
| FY 2023 - NET REVENUE RECONCILIATION CONSTANT CURRENCY | |||
| In thousands of euros | 2023 | 2022 | % Var |
| Reported Net Revenues | 6,591,977 | 6,063,967 | 8.7% |
| Variation due to Exchange Rate Effects | 133,610 | ||
| Net Revenues at Constant Currency | 6,725,587 | 6,063,967 | 10.9% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported Biopharma Net Revenues | 5,558,301 | 5,005,382 | 11.0% |
| Variation due to Exchange Rate Effects | 112,083 | ||
| Reported Biopharma Net Revenues at Constant Currency | 5,670,384 | 5,005,382 | 13.3% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported Diagnostic Net Revenues | 670,269 | 671,292 | (0.2%) |
| Variation due to Exchange Rate Effects | 16,517 | ||
| Reported Diagnostic Net Revenues at Constant Currency | 686,786 | 671,292 | 2.3% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported Bio Supplies Net Revenues | 159,957 | 146,076 | 9.5% |
| Variation due to Exchange Rate Effects | 2,655 | ||
| Reported Bio Supplies Net Revenues at Constant Currency | 162,612 | 146,076 | 11.3% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported Others & Intersegments Net Revenues | 203,450 | 241,217 | (15.7%) |
| Variation due to Exchange Rate Effects | 2,354 | ||
| Reported Other & Intersegments Net Revenues at Constant Currency | 205,804 | 241,217 | (14.7%) |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported U.S. + Canada Net Revenues | 3,898,961 | 3,855,607 | 1.1% |
| Variation due to Exchange Rate Effects | 88,993 | ||
| Reported U.S. + Canada Net Revenues at Constant Currency | 3,987,954 | 3,855,607 | 3.4% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported EU Net Revenues | 1,255,927 | 1,032,211 | 21.7% |
| Variation due to Exchange Rate Effects | 749 | ||
| Reported EU Net Revenues at Constant Currency | 1,256,676 | 1,032,211 | 21.7% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported ROW Net Revenues | 1,437,089 | 1,176,149 | 22.2% |
| Variation due to Exchange Rate Effects | 43,868 | ||
| Reported ROW Net Revenues at Constant Currency | 1,480,957 | 1,176,149 | 25.9% |
| FY 2023 - NET REVENUE RECONCILIATION CONSTANT CURRENCY EXCLUDING BIOTEST | |||
| In thousands of euros | 2023 | 2022 | % Var |
| Reported Net Revenues | 6,088,891 | 5,702,728 | 6.8% |
| Variation due to Exchange Rate Effects | 133,233 | ||
| Net Revenues at Constant Currency | 6,222,124 | 5,702,728 | 9.1% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported Biopharma Revenues | 5,055,215 | 4,644,143 | 8.9% |
| Variation due to Exchange Rate Effects | 111,706 | ||
| Reported Biopharma Net Revenues at Constant Currency | 5,166,921 | 4,644,143 | 11.3% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported U.S. + Canada Net Revenues | 3,897,511 | 3,853,488 | 1.1% |
| Variation due to Exchange Rate Effects | 88,993 | ||
| Reported U.S. + Canada Net Revenues at Constant Currency | 3,986,504 | 3,853,488 | 3.5% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported EU Net Revenues | 990,925 | 851,795 | 16.3% |
| Variation due to Exchange Rate Effects | 969 | ||
| Reported EU Net Revenues at Constant Currency | 991,894 | 851,795 | 16.4% |
| In thousands of euros | 2023 | 2022 | % Var |
| Reported ROW Net Revenues | 1,200,455 | 997,445 | 20.4% |
| Variation due to Exchange Rate Effects | 43,271 | ||
| Reported ROW Net Revenues at Constant Currency | 1,243,726 | 997,445 | 24.7% |
| FY 2023 - OTHER RECONCILIATIONS INCLUDING BIOTEST | |||
| In thousands of euros | 2023 | 2022 | % Var |
| Net Financial Debt | 9,416,312 | 9,191,000 | 2.5% |
| EBITDA Adjusted 12M | 1,484,650 | 1,287,000 | |
| Net Leverage Ratio1 | 6,3x | 7,1x | (11.1%) |
| (1) Excludes the impact of IFRS 16 | |||
| In thousands of euros | 2023 | 2022 | % Var |
| EBIT | 799,398 | 805,680 | (0.8%) |
| D&A | 451,759 | 415,339 | |
| EBITDA Reported | 1,251,157 | 1,221,019 | 2.5% |
| In thousands of euros | 2023 | 2022 | % Var |
| EBIT | 799,398 | 805,680 | (0.8%) |
| D&A | 451,757 | 415,339 | |
| Non-recurring costs2 | 223,009 | 25,866 | |
| EBITDA Adjusted | 1,474,164 | 1,246,885 | 18.2% |
| (2) Includes restructuring. divestment and transaction costs | |||
| In thousands of euros | 2023 | 2022 | % Var |
| EBIT | 799,398 | 805,680 | (0.8%) |
| D&A | 451,757 | 415,339 | |
| IFRS 16 | -101,784 | -99,990 | |
| Non-recurring Items3 | 335,276 | 166,174 | |
| EBITDA Covenant | 1,484,650 | 1,287,203 | 15.3% |
| (3) Non-recurring items are mainly related to transaction. restructuring and divestitures costs. as well as the amount of cost savings. operating improvements and synergies on a “run rate” | |||
| In thousands of euros | 2023 | 2022 | % Var |
|---|---|---|---|
| R&D recurrent expenses in P&L | 395.3 | 361.1 | 9.5% |
| R&D capitalized | 51.4 | 36.0 | 42.9% |
| R&D depreciation, amortization and write-offs | (64.7) | (43.9) | 47.5% |
| R&D CAPEX fixed assets | 2.1 | 0.9 | 138.0% |
| R&D external | (1.9) | (2.8) | (31.9%) |
| R&D net investment | 382.2 | 351.3 | 8.8% |
| In thousands of euros | 2023 | 2022 | % Var |
| PP&E additions | 246,430 | 291,676 | (15.5%) |
| Interest capitalized | (36,862) | (25,184) | 46.4% |
| CAPEX | 209,568 | 266,492 | (21.4%) |
| Note: for comparison purposes, figure reported in 2022 (EUR 297m) differs following a change of criteria in 2023 as software is not considered CAPEX anymore | |||
Annex XI. Glossary and abbreviations
Glossary and abbreviations
- Alpha-1 antitrypsin deficiency (AATD): inherited disease characterized by low levels or no alpha-1 antitrypsin (AAT) in the bloodstream. In its normal function, this protein is generated in the liver, released in the bloodstream and diffused to other organs such as the lungs.
- Albumin: the most abundant protein found in plasma (approximately 60% of human plasma). Produced in the liver, it is important in regulating blood volume by maintaining the oncotic pressure of the blood compartment.
- Alzheimer’s disease: the most common form of dementia, AD is an incurable, degenerative and terminal disease first described in 1906 by German psychiatrist and neuropathologist Alois Alzheimer.
- Anti-thymocyte globulin: blood serum with antibodies that bind with human T-cells, administered to patients before a stem cell transplant to destroy.
- ASFA: American Society for Apheresis, an organization of physicians, scientists and allied health professionals dedicated to promoting apheresis medicine for patients, donors and professionals through education, evidence-based practice, research and advocacy.
- Autoimmune disease: condition in which the immune system mistakenly attacks healthy cells.
- Babesiosis/Babesia virus: disease caused by microscopic parasites that infect red blood cells.
- Beta-amyloid: protein strongly implicated in Alzheimer’s diseases as the main component of certain deposits found in the brains of AD patients.
- Bullous pemphigoid: autoimmune disease that appears when the immune system attacks the skin and causes blisters, more common among the elderly.
- CIDP (chronic inflammatory demyelinating polyneuropathy): neurological disorder which causes gradual weakness, numbness, pain in the arms and legs, and difficulty in walking.
- Cirrhosis: medical condition resulting from advanced liver disease, characterized by generation of liver tissue by fibrosis (scar tissue) and regenerative nodules (lumps that occur due to attempted repair of damaged tissue).
- Cognitive impairment: alterations in thinking, learning, memory, judgment and decision making.
- COVID-19: infectious disease caused by a new coronavirus strain, with ”CO” short for corona, “VI” for virus and ”D” for disease.
- ELISA: enzyme-linked immunosorbent assay.
- EMA: European Medicines Agency.
- Factor VIII or FVIII: an essential blood clotting factor also known as anti-hemophilic factor (AHF). In humans, factor VIII is encoded by the F8 gene. Defects in this gene lead to hemophilia A, a sex-linked disease occurring predominantly in males. FVIII concentrated from donated blood plasma or recombinant FVIII (rFVIII) can be administered to hemophiliacs to restore hemostasis.
- Factor IX: an important blood clotting factor also known as Christmas factor or plasma thromboplastin component (PTC). It is one of the serine proteases of the coagulation system belonging to the peptidase family S1. In humans, a deficiency of this protein causes hemophilia B, a sex-linked disease that occurs predominantly in males.
- FDA: Food and Drug Administration, a U.S. health authority.
- Fibrin sealant: surgical adhesive material derived from plasma.
- Fibrinogen: coagulation factor found in human plasma crucial for blood clot formation.
- Fractionation: process of separating plasma into its component parts including albumin, immunoglobulin, alpha-1 antitrypsin and coagulation factors.
- GMP good manufacturing practice.
- GPO group purchasing organization.
- HAE (hereditary angioedema): Rare but serious genetic disorder characterized by recurrent episodes of severe swelling (angioedema), particularly of the face and airways, and abdominal cramping, caused by low levels or improper function of the C1- esterase inhibitor protein.
- HBV: hepatitis B virus.
- HCV: hepatitis C virus.
- Hematocrit: the percentage of red blood cells in the blood.
- Hematology: the study of blood, blood-forming organs and blood diseases.
- Hemoderivative: proteins obtained from the fractionation of human blood plasma (see plasma-derived proteins).
- Hemophilia: genetic deficiency characterized by the lack of one of the clotting factors, with two main variants:
- Hemophilia A: genetic deficiency of coagulation Factor VIII, which causes increased bleeding (more prevalent among males).
- Hemophilia B: genetic deficiency of coagulation Factor IX.
- Hemotherapy: treatment of a disease using blood, blood components and its derivatives
- HIV: human immunodeficiency virus.
- Hyperimmune globulins: type of immunoglobulins prepared in a manner similar to human normal immunoglobulin, except that the donor plasma has high titers of antibodies against an organism or antigen.
- IA: immunoassays, systems available in several formats to detect antibodies, recombinant proteins or a combination thereof.
- Intravenous: administration of drugs or fluids directly into a vein.
- Immunohematology: : branch of hematology related to the study of recombinant proteins and antibodies and their effects on blood and relationships between blood disorders and the immune system. Also referred to as transfusion medicine – blood bank, its main activities include blood typing, compatibility tests and crossmatching and antibody identification.
- Immunology: branch of biomedical science that covers the study of all aspects of the immune system in organisms, encompassing the physiological functioning of the immune system in states of both health and disease; malfunctions (autoimmune diseases, hypersensitivities, immune deficiency, transplant rejection) and the physical, chemical and physiological characteristics of the components of the immune system in vitro, in situ and in vivo.
- Immunoglobulin (IgG): plasma-derived proteins also known as antibodies that control the body’s immune response. They have multiple indications, with main uses including the treatment of: (i) immune deficiencies, (ii) inflammatory and autoimmune diseases and (iii) acute infections. IVIG is an immunoglobulin administered intravenously that contains IgG (immunoglobulin (antibody) G).
- ITP (chronic immune thrombocytopenia): autoimmune disorder in which patients produce antiplatelet autoantibodies and specialized white blood cells that destroy their blood platelets. This results in a low blood platelet count (thrombocytopenia) that may produce bruising or excessive bleeding
- IVD: in vitro diagnostic.
- IV solutions/intravenous solution: medicine or homogeneous mixture of a substance in liquid, enabling its infusion into the circulatory system through a needle.
- Lipemic plasma: plasma with a cloudy and/or milky appearance caused by excess lipids (hyperlipidemia) due mainly to cholesterol and/or triglycerides in the blood.
- MRB: Marketing Research Bureau.
- Molecular diagnostic: discipline that studies genomic (DNA) and proteomic (proteins) expression patterns using information to distinguish between normal, precancerous and cancerous tissues at the molecular level.
- Monoclonal antibody (mAb): antibody produced by a single clone of cells typically used in immunotherapy (i.e. treatments of autoimmune or inflammatory disorders and cancer); diagnostic testing; cell identification; and tracking. Monoclonal antibodies are a cornerstone of immunology and becoming increasingly prevalent as therapeutic agents.
- Myasthenia gravis (MG): chronic autoimmune, neuromuscular disease that causes weakness in the skeletal muscles which worsens after periods of activity and improves after periods of rest. These muscles are responsible for functions involving breathing and moving parts of the body.
- NAT: nucleic acid amplification testing.
- Neurology: science that deals with the anatomy, functions and organic disorders of nerves and the nervous system.
- North America: United States and Canada.
- Ophthalmology: branch of medicine and surgery that deals with the diagnosis and treatment of eye diseases.
- Pandemic: worldwide spread of a new disease.
- Parkinson’s disease: complex neurodegenerative disorder characterized by different combinations of motor and non-motor symptoms for each patient.
- PCR: polymerase chain reaction, a method widely used to rapidly make millions to billions of copies of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it to a large enough amount to study in detail.
- pdFVIII: plasma-derived Factor VIII.
- Pharmacovigilance: practice of monitoring the effects of medical drugs after they have been licensed for use, especially to identify and evaluate previously unreported adverse reactions.
- Plasma: yellow-hued liquid part of the blood comprised by numerous proteins in solution.
- Plasma-derived proteins: purified plasma proteins with therapeutic properties obtained through the fractionation of human plasma. Albumin, immunoglobulins, factor VIII and alpha-1 antitrypsin are the main plasma proteins.
- Plasma proteomic:high-throughput analysis of plasma biomarkers using very powerful and sensitive specialty instruments.
- Plasmapheresis: technique by which plasma is separated from other blood components such as red blood cells, platelets and other cells. These unused blood components are suspended in saline solution and immediately reinjected back into the donor. Since donors only provide plasma as opposed to whole blood, the recovery process is faster and better tolerated, enabling greater frequency of donations. Developed by José Antonio Grifols Lucas in 1951, plasmapheresis is the only procedure capable of obtaining sufficient quantities of plasma to cover the manufacturing needs for plasma protein therapies.
- Pneumology: specialty focused on the diagnosis and treatment of respiratory diseases and conditions, from asthma to tuberculosis.
- PPTA: Plasma Protein Therapeutics Association.
- Primary arthroplasty: surgery performed to replace damaged joints with artificial joints or prostheses, used in cases of hip fractures, osteoarthritis and other rheumatic diseases.
- Primary immunodeficiency: inherited condition affecting one or more areas of the immune system characterized by an impaired immune response, weakening the immune system and increasing the likelihood of infections and other health problems.
- Prolastin®/ Prolastin®-C: concentrated form of alpha-1 antitrypsin (AAT) derived from human plasma and approved only for chronic replacement therapy in people with genetic AAT deficiency. Administered as prescribed, Prolastin raises the levels of AAT in the blood and lungs, which may help reduce the damage to the lungs caused by destructive enzymes.
- Proteome: complete set of proteins expressed by an organism that determine an organism’s nature, bodily functioning and behavior.
- Recombinant: protein prepared by recombinant technology, coded by the manipulated gene, with procedures used to combine segments in a cell-free system (an environment outside a cell organism). Known as highly potent medicines, they avoid off-target side effects and take a shorter time to develop than small molecules.
- Recovered plasma: plasma derived from whole blood collected in blood donations
- rFVIII: recombinant Factor VIII, the antihemophilic factor A obtained using recombinant DNA technology. Using this technology, pure factor is synthesized in the laboratory instead of being extracted from blood plasma.
- Rh (Rhesus) blood group system : the most important blood group system after ABO, the Rh blood group system consists of 50 defined blood-group recombinant proteins, among which the five recombinant proteins D,C, c, E and e are the most important. The commonly used terms Rh factor, Rh positive and Rh negative refer to the D antigen only.
- ROW: rest of the world.
- SARS-CoV-2:: severe acute respiratory syndrome coronavirus 2, the coronavirus strain that causes coronavirus disease 2019 (COVID-19).
- Secondary immunodeficiency: compromised immune system due to an environmental factor such as HIV, chemotherapy, severe burns or malnutrition.
- SCIG: subcutaneous immunoglobulin.
- Single-cell transcriptomics: technique to characterize cell identity.
- SubQ: sub-cutaneous.
- Thrombin: enzyme that presides over the conversion of fibrinogen to fibrin, which promotes blood clotting.
- Transfusion medicine: branch of medicine that encompasses immunohematology, blood and plasma screening, and blood typing, among others.
- West Nile virus (WNV): mosquito-transmitted virus. Humans are mainly infected through mosquito bites, but infection may also occur through organ transplantation and blood.
- Von Willebrand disease (vWD): the most common hereditary coagulation abnormality described in humans, although it can also be acquired as a result of other medical conditions. It arises from a qualitative or quantitative deficiency of von Willebrand factor (vWF), a multimeric protein required for platelet adhesion.
- Zika virus: infectious disease spread by the bite of an infected Aedes species mosquito
Independent Review Report
Independent Review Report



