Guarantee the supply of plasma and promote countries’ self-sufficiency to expand access to plasma-based treatments, while upholding our globally recognized standards of quality, safety, transparency and engagement.
Our roadmap. Grifols 2030 Agenda
- Increase product donations to patient programs
- Increase donations of clotting factors in developing countries
- Boost product donations for emergency relief efforts
- Achieve “excellent” or “good” service ratings from donors
- Encourage more donors to recommend the donation process to family and friends
- Increase ratings on donor applications
Priorities
- Donors
- Patients
- Patient
associations - Access to treatment and self-sufficiency
WE SUBSCRIBE TO THE PRINCIPLES OF BIOETHICS

AUTONOMY
Each person is able to make decisions freely and independently.
JUSTICE
Healthcare resources are allocated equitably and fairly.
BENEFICENCE
We work to optimize benefits for patients
and diminish potential harm.
NON-MALEFICENCE
Our actions cannot intentionally create a harm or injury to the patient.




Serving as a bridge between donors and patients
Grifols transforms donor plasma into life-enhancing medicines, ensuring responsible operations at every stage of the value chain.
We need nine to 12 months to transform plasma into plasma-based medicines
Donors
920,000+
donors
2,579 M$
Positive impact on donors
plasma
55% of whole blood
90% of water
7% are essential proteins
patients
800,000+
patients treated
27,370 M$
positive impact on patients
- Dozens of conditions are treated with plasma therapies
- Proactive promotion of access to treatment
- Numerous patient support programs
More information: “A Differential Value Chain” section. Cadena de Valor
We work to guarantee the procurement of plasma
Awareness
Campaigns and collaborations in the U.S. and Europe.
Support for International Plasma Awareness Week (IPAW), organized by the Plasma Protein Therapeutics Association (PPTA).
Outreach with local communities, policymakers, and patient associations.
Action
Promote science-based policies to increase plasma donations around the world:
– Support EU policies that encourage strategic plasma self-sufficiency: new Substances of Human Origin (SoHO) regulation in Europe.
– Expand funding for the U.S. Health and Human Services plasma-awareness campaign.
– Promote the Congressional Plasma Caucus, formed by U.S. legislators who aspire to raise awareness of the critical importance of plasma therapies and plasma donations.
– Eliminate state regulatory barriers that hinder the operations of U.S. plasma donation centers.
More information: “Corporate Governance”
Plasma centers
Grifols has the world’s largest private network of plasma centers.
Global and diversified presence
More information on the network of plasma centers: “About Grifols”
Self-sufficiency
In Egypt, first plasma-based products manufactured with Egyptian plasma.
Agreement signed with Canadian Plasma Resources (CPR) to open plasma centers in Canada as part of Grifols’ alliance with Canadian Blood Services.
More information: “Access to Treatments”
Our plasma supply platform encompasses integrated plasma centers within our network and strategic collaboration agreements with third parties
Our commitment to donors
Respect for people’s intrinsic dignity and human rights is a cornerstone of all Grifols’ activities, aligning with the core principles of the Universal Declaration of Human Rights (1948), Declaration of Helsinki (1964), and UNESCO Universal Declaration of Bioethics and Human Rights (2005).
As outlined in Grifols’ Code of Conduct, all company interactions with stakeholders, including donors, are grounded on a fundamental respect for human rights. This principle is articulated in Grifols’ Donor Policy, which stresses the need to respect country-specific legal regulations, ensure non-discrimination, and implement measures to protect donors’ health and safety.
Grifols provides clear and reliable information for donors at every stage of the donation process, and prior informed consent is mandatory.
8 commitments
Safeguard donors’ health, safety and well-being.
Respect donors’ human rights and ensure equal treatment following the principles of non-discrimination.
Ensure donors provide informed consent before donating plasma.
Respect legislation in each country regarding donor compensation and the frequency of plasma donations.
Support local communities where donor centers are located.
Comply with personal data legal requirements and implement all necessary measures to protect donors’ privacy and personal data.
Promote open lines of communication and awareness about the benefits of plasma medicines.
Ensure every interaction with donors is professional, respectful, helpful and engaging.
Access to the “Plasma Donor Policy”
Access to the Code of Conduct
Grifols donors represent a cross-section of society
Age
Balanced distribution
44%
Women
56%
Men
Education and employment
62%
college graduates
11%
high school graduates
26%
current university students
95%
full-time employees
In 2023, Grifols surveyed over 1,300 qualified U.S. plasma donors to learn their primary incentives for donating plasma, among other issues. While donors cited financial compensation as the motivating factor for their first donation, they said altruism and the service and care given at Grifols donation centers were what turned them into frequent donors.
The plasma donation process is safe and its collection is highly regulated.
73%
of donors who left reviews in Grifols’ plasma centers*, assigned a top review
*Grifols plasma donation centers
Donors and donations
Donor regulations
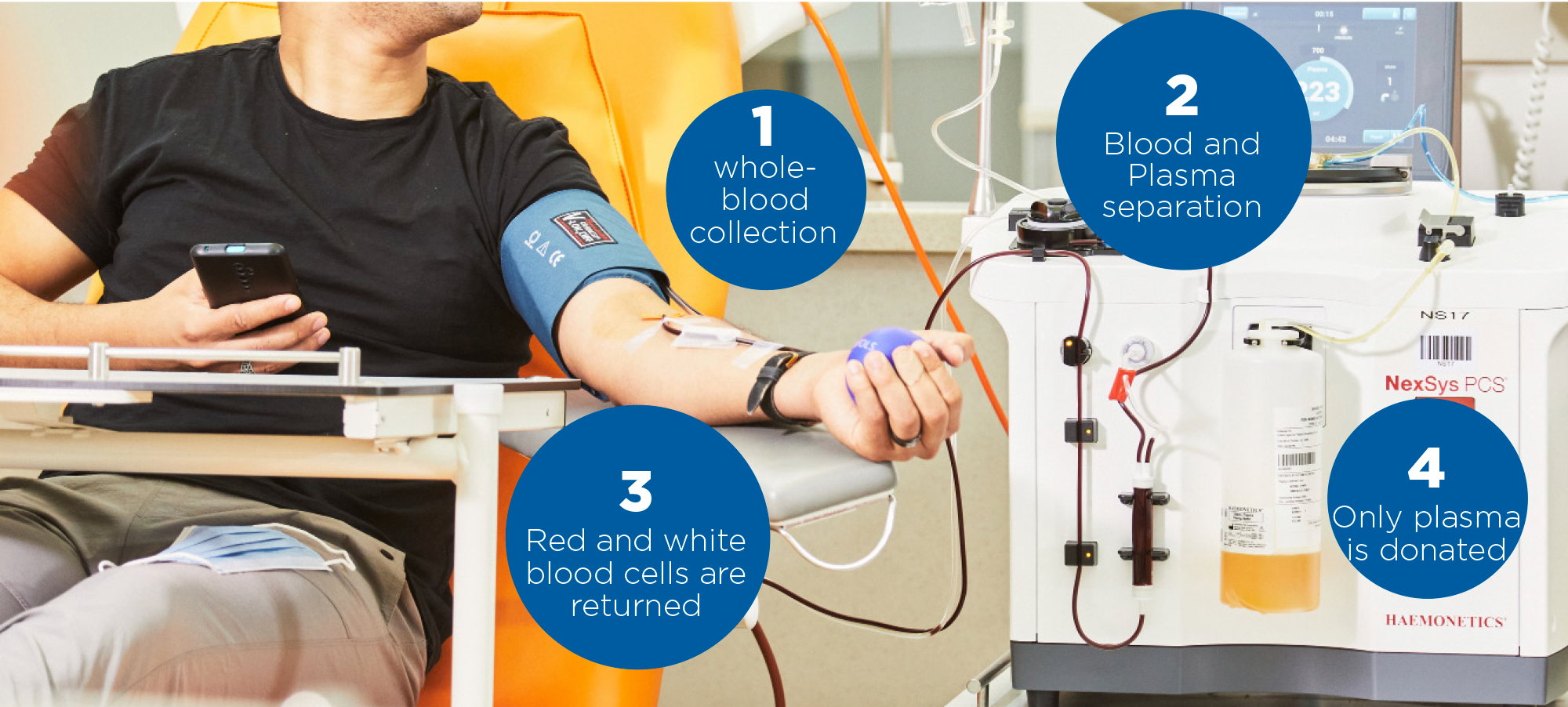
Plasma is procured from whole blood donations (recovered plasma) or through plasmapheresis (sourced plasma), a specific technique for plasma donation developed by José Antoni Grifols i Lucas.
Plasma collection for the manufacture of plasma-based medicines is subject to strict regulations by global healthcare authorities and good manufacturing practices (GMP). The Food and Drug Administration (FDA) is the maximum health authority in the United States, while in Europe, the European Agency for Medicine (EMA) oversees this function. The Plasma Protein Therapeutics Association (PPTA) defines and monitors additional quality standards as part of its voluntary IQPP (International Quality Plasma Program) certification.
Donating plasma is extremely safe, with few or no side effects. Using the plasmapheresis technique, plasma is extracted from whole blood, and blood cells, platelets and other components are returned to the donor. The body regenerates the volume of collected proteins in about 48 hours, in contrast to a two-month regeneration time for red blood cells obtained from whole blood donations.
In 2023, Europe developed a new regulation to ensure the safety and quality of substances of human origin (SoHO), including plasma donations. This directive aims to improve access to SoHO therapies, which play acritical role in the healthcare systems of all EU Member States.
More information on related FDA regulations
More information of the SoHO Regulation and agreements signed
Quality control in Grifols donation centers
Grifols’ plasma donation centers adhere to the highest quality and safety standards while also undergoing routine regulatory inspections to guarantee donor safety and the quality of donated plasma. In 2023, Grifols has not received any administrative action in plasma centers due to suspension, revocation or loss of any license or certification; warning letter, imposed suspension of any regulated activity.
| Inspecciones regulatorias en los centros de plasma | |||
| Días de inspección | 2023 | 2022 | 2021 |
| FDA* | 137 | 119 | 80 |
| EMA | 196 | 182 | 196 |
| CLIA-COLA | 169 | 108 | 145 |
| PPTA | 97 | 123 | 117 |
| TOTAL | 599 | 532 | 538 |
Includes Biotest.
* More than 95% of FDA inspections resulted in zero observations.
Plasmapheresis, a safe procedure for donating plasma
Conditions for donating plasma
Grifols follows all the regulatory requirements of global health authorities and comprehensive evidenced-based processes to establish peoples’ eligibility for donating plasma. Donors must postpone the donation process if medical exams reveal abnormal levels or irregular parameters to exclude the possibility of an underlying health issue. These biomarkers include:
- Irregular heartbeat
- High body temperature
- High or low hematocrit
- High or low total protein
- Lipemic plasma
Grifols safeguards donors’ health
Grifols only uses plasma from qualified donors and never from occasional donors. Once qualified, donors undergo annual physical exams and thorough assessments of their medical, surgical and travel history, in addition to medical-history evaluations every time they donate.
This information is registered in the donor’s file and treated confidentially in line with Grifols’ Global Privacy and Data Protection Policy principles. Before each donation, a specialized Grifols staff member checks the donor’s vital signs and weight, as well as blood and plasma protein levels to confirm they can safely donate. In this way, Grifols monitors donors’ overall health and well-being, a long-standing corporate priority.
Plasmavigilance
As in previous years, Grifols’ U.S. plasmavigilance data in 2022 revealed minimal donor adverse events (DAE)*, with side effects in only 0.3% of donations. Most adverse effects were minor, resulting in hypotensive events or phlebotomy-related injuries like hematomas. Severe reactions requiring medical assistance were extremely rare, representing only 0.008% of Grifols’ total donations.
Data on donor side effects continues to confirm the safety of plasma donation.
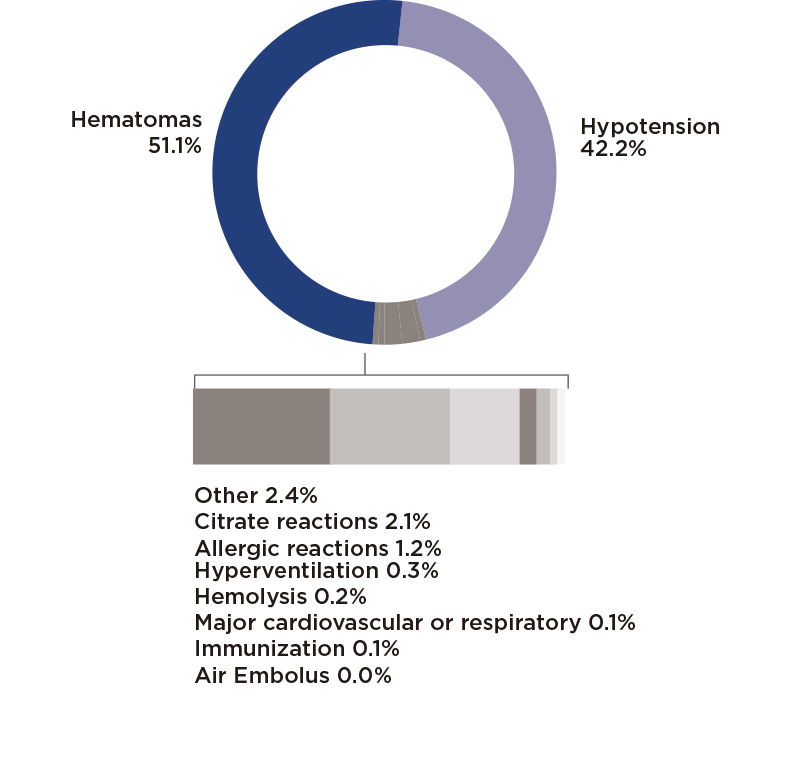
* Plasma surveillance data in 2022 according to the DAE categorizations established by the PPTA (Plasma Protein Therapeutics Association) IQPP Standard for reporting donor adverse events. This data is published with a one-year lag according to the required reporting cycles.
Eligibility requirements to donate plasma
Qualified donors
Donate at least 2 times within 6 months
Maximum 2 times every week
Between 18-69 years old
+50 kg
Medical examination with normal levels
/p>Documentation
Valid picture ID: driver’s license, passport, etc.
Proof of Social Security Number
Proof of address
Donor health screening
Weight
Blood pressure
Pulse
Temperature
Anemia
Protein levels
Every donation is treatedn
VHC, VHB, VHA, VIH and B19 virus detection
Screening for hepatitis B, hepatitis C and HIV antibodies
Other routine tests
In 2023, Grifols worked to implement the FDA Individual Risk Assessment guidance for evaluating donor eligibility into its donor health questionnaire by early 2024, although many of Grifols’ current criteria are even stricter.
Access to more information about:
Plasma donations in Europe: Haema, Plasmavita, Biotest
Plasma donations in Egypt
Protecting donors’ health is our top priority
As part of its commitment to donor health and safety, Grifols directly supports the research by diverse scientific institutions and associations to gain a deeper understanding of the potential effects of plasmapheresis on donors’ health.
Donations and donor health
Regular donations have no adverse effects
Published in Transfusion magazine in 2023, this transversal study was conducted by the Plasma Protein Therapeutic Association (PPTA) to determine if plasma donation at FDA-defined frequency and volume levels has an impact on donor health. Donors from 14 U.S. plasma donation centers, including several Grifols plasma donation centers, took part in the study, which concluded that paid plasma donations at these levels are consistent with donor health and well-being. Even at the highest frequency, plasmapheresis alone has no associated negative health effects.
Study: Effects of donation frequency on U.S. source plasma donor health
Plasmavigilance study in the U.S.
The rate of side effects from plasma donations via plasmapheresis is insignificant
More than 1.1 million donors, who collectively account for 72% of the U.S. source plasma collected over a four-month period, took part in the first industry-wide, multi-company study on the incidence, frequency and type of adverse effects of plasmapheresis. Promoted by the PPTA, in cooperation with various industry firms, the study confirmed the overall safety of plasmapheresis.
Following FDA standards of collection volumes and donation frequency, the rate of adverse events (AE) was 1.58 per 10,000 donations. Moreover, 90% of AEs were minor, such as hypotension and phlebotomy-related hematomas, with no reports of serious or severe adverse events. The study’s findings were published in 2021 in the scientific journal Transfusion.
Study: Plasmavigilance: Source plasma joins the call to arms
Cholesterol levels
Research findings suggest a decline in cholesterol levels
Apheresis or low-density lipoprotein extraction is used to treat patients with familial hypercholesterolemia. In some donors, the low-volume plasmapheresis used in plasma donations may also lower cholesterol levels. This research analyzed the effect of plasmapheresis on total LDL and HDL cholesterol levels among healthy plasma donors, concluding that total and LDL cholesterol levels may decline in donors with elevated baseline cholesterol levels following regular voluntary plasmapheresis. In donors with low baseline HDL levels, HDL cholesterol levels may increase.
Iron levels
Plasma donation has no effect on iron reserves
This study found no loss of iron or decline in ferritin levels because of regular plasma donations – even in the case of long-term donors – as opposed to whole blood donations. These findings deem it unnecessary to monitor donors’ iron levels or recommend iron supplements.
Blood pressure
The results suggest a beneficial effect for donors with high blood pressure
Grifols led a study to discern the potential effects of plasmapheresis on blood pressure, finding a beneficial effect among donors with high baseline blood-pressure levels, whose systolic and diastolic blood pressure decreased significantly when their donation intervals are under 14 days. No decline in blood pressure was observed among donors with normal baseline blood pressure levels.
Study: The effect of plasmapheresis on blood pressure in voluntary plasma donors
Reasons to stop donating
Health reasons, either real or perceived, are not main motivating factors to stop donating
In 2023, Transfusion published the results of a study to discern donors’ rationale when deciding to no longer donate plasma. The survey was conducted among donors in 14 plasma donation centers of several companies, Grifols included, who had stopped donating for at least six months. Lack of time (30.2%), insufficient compensation (14.7%) and procrastination (14.3%) were among the most common reasons cited, showing that real or perceived negative health impacts generally were not primary drivers of their decision to stop donating.
Donation centers in committed communities
Grifols’ U.S. plasma donation centers are located throughout the country, with no particular concentration in specific areas.
When evaluating suitable plasma-center sites, Grifols considers areas with a solid commitment to community progress, active chambers of commerce, and a strong vocation to advancing social progress. For Grifols, a community’s active participation in the plasma donation process is paramount to securing patients’ access to life-sustaining plasma-based treatments.
Grifols’ employees work proactively to forge ties with community residents by organizing educational, social and awareness-raising events on the vital need for plasma donations. Plasma centers also collaborate with local businesses and non-governmental organizations to raise awareness on plasma and the manufacturing process of plasma treatments.
The company considers other criteria when choosing communities for its plasma donation centers, including low viral markers, below-average crime statistics and community heterogeneity, which is critical to ensuring a diverse donor pool.
More information on value creation by Grifols plasma donation centers: “Sustainable Growth”
More information on our social action with donors: “Impact on Society”
Our commitment to patients
1. Safety and quality
- Offer the best possible therapies, products and services through continuous innovation and leadership in safety and quality standards
2. Transparency and independence
- Engage and support of patients and organizations by serving as a reliable and transparent source of information
3. Access to treatment
- Advocate and advance the principles of justice and equality in health care, with special emphasis on increasing access to plasma therapies.
Patient notification system
Grifols has supported and participated in the Plasma Protein Therapeutics Association’s (PPTA) Patient Notification System (PNS) since 1998. This system is free of charge, confidential and exclusively offered to patients and registered users, who receive notifications regarding the voluntary or mandatory withdrawal of plasma medicines.
Grifols subscribes to international principles
- International Bill of Human Rights (includes the Universal Declaration of Human Rights, International Covenant on Civil and Political Rights, and International Covenant on Economic, Social and Cultural Rights).
- Declaration of Helsinki.
- UNESCO Universal Declaration on Bioethics and Human Rights.
- United Nations Guiding Principles on Business and Human Rights.
- OECD Guidelines for Multinational Enterprises.
- United Nations Global Compact.
We produce life-enhancing medicines
An estimated two million people in Europe1 suffer from one of the 12 most common rare diseases, including hemophilia and primary immunodeficiency (PIDD), which may be treated and managed with plasma-derived therapies.
At the same time, scientific advances continue to broaden the range of high-prevalence diseases that could benefit from plasma-based therapies. Plasma proteins are also used in everyday medical treatments, emergency services and surgical interventions, among other uses.
Diseases and conditions treatable with plasma-based medicines2
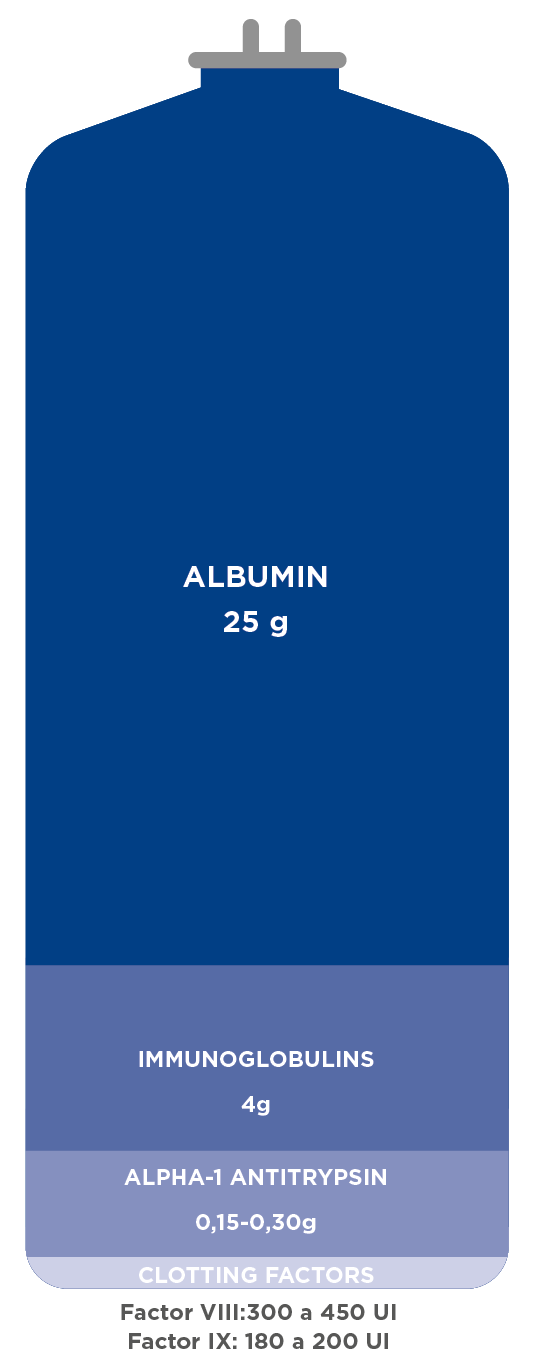
ALBUMIN
– Liver cirrhosis
– Surgery (cardiac and major)
– Intensive care (e.g. sepsis, burns)
IMMUNOGLOBULINS
– Immunodeficiencies
– Primary (PIDD)
– Secondary (SID)
– Neurological conditions
– Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP)
– Acute demyelinating polyneuropathy (Guillain Barré)
– Multifocal motor neuropathy (MMN)
– Hematological conditions
– Immune thrombocytopenia (immune thrombocytopenic purpura or ITP)
– Neuromuscular diseases
– Myasthenia Gravis (MG)
– Post-exposure prophylaxis for rabies
– Post-exposure prophylaxis and treatment for tetanus
– Immunoprophylaxis of hepatitis B
ALPHA-1 ANTITRYPSIN
– Alpha-1 antitrypsin deficiency disorder
CLOTTING FACTORS
– Bleeding disorders
– Hemophilia A and B
– Von Willebrand disease (VWD)
– Rare clotting factor deficiencies
– Trauma/injury-related hemorrhaging
– Overdose of anticoagulants or toxic substances that induce bleeding
(1) Silvia Rohr and Rianne Ernst, “Key Economic and Value Consideration for Plasma-Derived Medicinal Products (PDMPs) in Europe,” PPTA.
(2) This information does not assume that Grifols’ products have the necessary regulatory approvals to treat the aforementioned indications.
| Inspecciones regulatorias en los centros de plasma | |||
| Días de inspección | 2023 | 2022 | 2021 |
| FDA* | 137 | 119 | 80 |
| EMA | 196 | 182 | 196 |
| CLIA-COLA | 169 | 108 | 145 |
| PPTA | 97 | 123 | 117 |
| TOTAL | 599 | 532 | 538 |
** General information on the benefits of plasma-based therapies.Source: PPTA
More information: How plasma-derived medicines boost health value
Plasma-derived medicines may offer significant and lifelong benefits to patients, increasing their life expectancy and quality of life, while reducing the risk of life-threatening complications among those with plasma-protein deficiencies. For this reason, most plasma-derived medicines are designated as essential medicines for adults and children by the World Health Organization, while numerous others are included on the EU essential medicines list.
Access to treatment and diagnosis
Program to promote countries’ self-sufficiency in plasma and plasma-derived medicines: leading the change
The World Health Organization (WHO), the Council of Europe and other institutions have stressed the urgent need for countries to increase their self-sufficiency in plasma medicines to ensure patients have adequate access to these life-sustaining treatments.
As per the WHO1 resolution WHA 63.12, Member States should “take all the necessary steps to establish, implement and support nationally-coordinated, efficiently-managed and sustainable blood and plasma programmes according to availability of resources, with the aim of achieving self-sufficiency.” According to the World Health Organization , only 65 of the 171 reporting countries fractionate nationally collected plasma to produce plasma-derived medicines, and in 91 countries, plasma-based medicines are imported.
Grifols supports and collaborates with countries to increase their levels of self-sufficiency as part of its ongoing efforts to promote and improve access to treatment. The company leads this change through the Grifols Self-Sufficiency Program, reinforcing national healthcare systems and lessening their dependence on third parties.
1. https://www.who.int/es/news-room/fact-sheets/detail/blood-safety-and-availability
Advances in the strategic alliance in Canada
Grifols reached a long-term collaboration agreement with Canadian Blood Services (CBS) in 2022 to accelerate the country’s immunoglobulin (Ig) self- sufficiency from 15% to 50% in the shortest timeframe possible, reducing the volume of plasma-medicine imports.
In 2023, Grifols made further inroads in meeting the needs of Canadian patients by bolstering its vertically integrated supply chain, comprised by new donation centers and the Montreal production facilities. Production will take place at Grifols’ Clayton facilities (North Carolina, U.S.) until the Montreal facility is fully operational in 2027.
Increasing Egypt’s self-sufficiency
In 2020, Grifols began developing the first integrated platform in the Middle East and Africa to supply plasma therapies at national and regional levels as part of its strategic alliance with the Egyptian government. Through this collaboration, the company will promote Egypt’s self-supply of plasma medicines through a pioneering public-private partnership.
Grifols Egypt currently operates nine plasma centers, as well as analysis and storage facilities that employ 625 people. The company plans on opening a total of 20 centers. Meanwhile, it continues to oversee the construction of a plasma fractionation plant, purification plant and other installations, expected to be operational in 2025. Until then, all plasma collected (up to 1 million liters per year) will continue to be processed in Spain and returned to Egypt as finished product.
In 2023, Grifols Egypt received the first medicines made with Egyptian plasma: immunoglobulins, factor VIII and albumin. Thanks to the upturn in national donations, Egypt will be self-sufficient in immunoglobulins in 2024, and albumin and factor VIII, in 2025.
Direct initiatives to support patients
Grifols actively works to promote availability to its essential treatments, especially when unforeseen circumstances may affect or limit its access. Since 2006, Grifols has led initiatives to support patients in the U.S. during lapses in their insurance coverage. The company also supports patients by providing treatment access to those who require temporary assistance and comprehensive programs to help them better manage their disease.
World Hemophilia Organization
An estimated 400,000 people around the world suffer from severe hemophilia, yet 75% remain untreated. To address this issue, Grifols began collaborating with the World Federation of Hemophilia (WFH) Humanitarian Aid Program in 2014, donating clotting factors for hemophilia patients in need of treatment. Grifols’ donations also support the WFH’s Global Alliance for Progress (GAP) program. In its second decade, this initiative aims to increase the number of patients diagnosed and treated for bleeding disorders, especially in developing countries.
In 2023, Grifols donated more than 2.8 M IU to the Syrian Hemophilia Society following the earthquakes in Turkey and Syria, providing treatment for hemophilia patients who were seriously injured in the affected areas.
![]()
Patients treated from 2014-2023*
8,861
Patients treated in 2023*
1,693
Countries
33
Million IU** donated in 2023
4.7
*Source: WFH data/ **IU = international units
Emergency aid
Grifols provides medical resources to healthcare professionals in the aftermath of natural disasters, extreme poverty and other humanitarian emergencies in collaboration with Direct Relief, a humanitarian relief organization present in more than 80 countries. In all cases, the company does its utmost to guarantee the rapid availability of donated product.
In 2023, Grifols also collaborated with Lebanon, where an economic crisis has led to a widespread shortage of medicines. The company donated 2,100 vials of factor VIII, equivalent to six months of treatment for 350 hemophilia patients, to the humanitarian aid organization Anera.
![]()
Value of medicines donated from 2019-2023
2.7 million
Value of medicines donated 2023
0.7 million
Patients treated in 2023
+16,000
Units of products donated in 2023
+23,000
Support for AADT patients
AlfaCare is a holistic support program for alpha-1 antitrypsin deficiency (AATD) patients, offering training, emotional support and resources to help them better manage their condition by promoting new habits and initiatives to enhance their physical and psychological well-being. The program was launched in Spain in 2018 with the collaboration of the Alpha-1 Spain Association and the support of a multidisciplinary clinical team, including psychologists and patient mentors. Since then, it has expanded to Germany under the name AlphaCare and to Italy as GriCare.
AlfaCare has been proven as a high-value resource for AADT patients. As of December 2023, it supports 265 patients in Spain, who receive psychological support and respiratory physiotherapy, among other services. Among these patients, 32 benefit from at-home infusions. Outside Spain, the initiative supports 712 patients in Germany and 88 patients in Italy.
![]()
AlfaCare Program
1.000+
patient beneficiaries in 3 countries
More information on AlfaCare: www.grifols.com
Enhancing diagnostics
Safe transfusions
Grifols supports the integrated strategy promoted by the WHO in the realm of specialized diagnostics. Through its Diagnostic unit, the company works to increase the availability of NAT screening tests in blood banks to detect human immunodeficiency virus (HIV), hepatitis B and C, and emerging viruses such as babesiosis, the Zika virus and the West Nile virus.
In parallel, the company strives to extend transfusion diagnostic solutions in lower-middle-income countries1, including the Philippines, India, Egypt and Indonesia. According to the WHO, 50% of donated blood is collected in lower-middle or low-income countries , which account for 80% of the world population. Many of these countries lack basic measures to guarantee safe transfusions. This is also the case in China, where Grifols collaborates with Shanghai RAAS to progressively raise transfusion safety standards in the country’s donation centers.
As of 2023, over 38 million blood donations had been tested using Grifols NAT technology and over 42 million blood-typing gel cards had been supplied.
1.https://datos.bancomundial.org/nivel-de-ingresos/paises-de-ingreso-bajo
First free and patient-direct program to detect AADT
In 2023, Grifols launched the AlphaID At Home Genetic Health Risk Service, the first free, direct program for U.S. residents to assess their genetic risk of alpha-1 antitrypsin deficiency (AATD). With symptoms similar to COPD, AATD affects an estimated one in 2,500 Americans and may cause lung disease and liver disease.
Using the innovative AlphaID™ oral test, people can detect their risk of AATD through a saliva sample, with no need to visit a healthcare professional.
By 2023, dozens of people have benefited from both the AlphaID At Home in the U.S. and the Alpha ID kit in many other countries enabling the detection of DAAT and helping patients to take the appropriate measures to address this health problem.
Grifols is also working to develop new diagnostic tests for personalized medicine for the prognosis, response prediction and monitoring of biological drugs, as well as novel molecular diagnostic and prognostic tests in oncology, autoimmunity, cardiovascular and central nervous system medicine.
Patient associations
Patient associations and advocacy groups play a fundamental role in global healthcare systems by giving patients a voice. At Grifols, they form an essential part of the firm’s decision making, with actions coordinated and managed by the Global Patient Affairs team.
The company’s interactions with patient associations respect country-specific regulations and transparency principles. Grifols also has standardized operating procedures to establish eligibility, compliance, ethics and transparency guidelines for all of its collaboration agreements, grants and donations. These criteria are defined in the Patient and Patient Organizations Policy.
Grifols publishes country-specific reports on its contributions to global patient organizations.
More information on Grifols’ contributions to patient groups
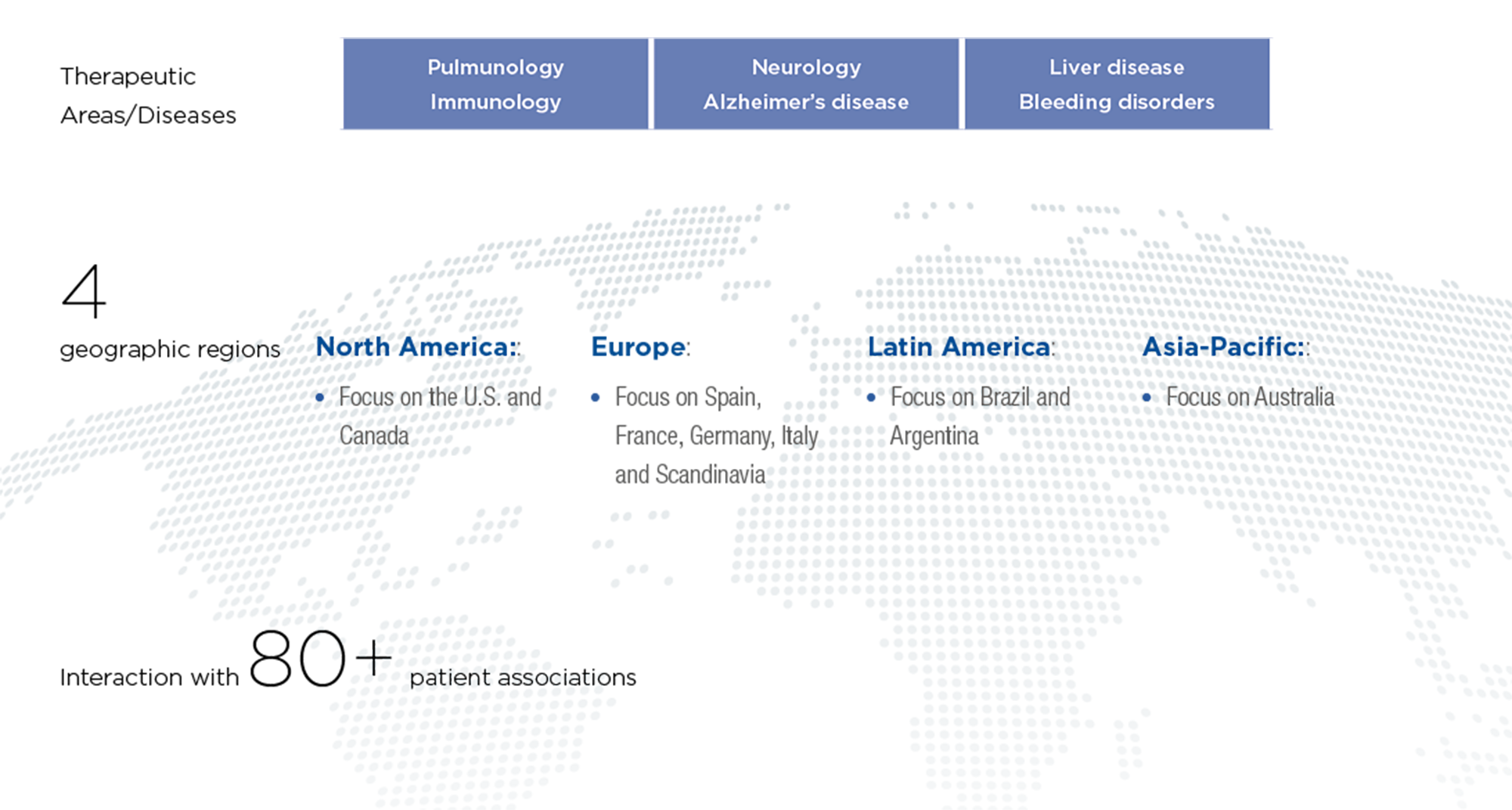
Broad scope in 2023
Grifols interacts with more than 80 global patient organizations in core therapeutic areas. In 2023, the company allocated more than EUR 16 million for product donations and resources to support nearly 60 of patient associations and their diverse programs and activities. The company has focused on Europe to increase patient organization involvement.
How Grifols collaborates
- We educate patients and patient organizations about the unique nature of plasma therapies and the complex processes to produce them.
- We advocate side-by-side with organizations to improve access to life-enhancing plasma therapies.
- We engage with patient communities as trusted source of information and expertise on plasma therapies.
- We support patient organizations through volunteer efforts and financial resources in accordance with relevant laws and regulations.
Guiding principles of Grifols’ patient interactions
- Mutual benefit: Demonstrate a clear benefit for patients
- Transparency: Public disclosure of financial contributions to patient associations and encouragement that they do likewise
- Integrity: Commitment always in alignment with corporate objectives and priorities
- Compliance: Compliance with all legal norms, rules and guidelines, as well as Grifols policies
- Independence: The right not to support Grifols’ actions
See Policy on Patients and Patient Organizations
See details on Grifols’ contributions to patient advocacy groups
Collaborations and programs
Donation programs to patient associations
Grifols supports projects and initiatives developed by patient organizations in four core areas:
- Education and empowerment: Efforts to involve patients in making decisions regarding their health. In the case of rare diseases, training medical professionals is also key to reduce the time to diagnosis and improve the approach to these conditions. To this end, Grifols collaborates in various seminars and scientific conferences.
- Greater awareness and visibility: Initiatives to give visibility to patient communities and commemorate their related International Days to forge community ties and help get their needs and challenges included on political agendas. Grifols takes part in creating and maintaining different communication channels and informational collateral.
- Patient experience and welfare: Grifols collaborates with projects aimed at improving disease management and patient experience, including programs to facilitate the administration of treatments and promote a healthy lifestyle and nutritional habits, among others. In 2023, the company supported Spanish hemophilia associations by offering physiotherapy services to address patients’ musculoskeletal challenges and functional capacity, as well as other associations to provide psychological support programs for pediatric and adult patients.
- Advocacy and access: Patient organizations work to ensure equity in access to treatment, and in the case of plasma treatments, to ensure there is sufficient plasma. The shortage of plasma-based medicines continues to be an urgent global challenge. In 2023 Grifols continued to support various plasma awareness and education campaigns to increase donations, especially in view of the SoHO review by the European institutions. These initiatives were also launched by associations of other pathologies, such as primary immunodeficiency or alpha-1 antitrypsin deficiency.
Examples of programs and initiatives
- Supporting patients’ needs
In Spain, Grifols supports the Spanish Association of Primary Immune Deficiencies (AEDIP), which is leading the “Spanish Consensus for the Sufficiency of Plasma and its Derivative Treatments”. This group is working to promote a national strategy for plasma and plasma-derived treatments which promotes far-reaching solutions to make Spain a benchmark in the collection, management and use of plasma. Therefore, guaranteeing the sufficiency of medicines for patients.
- Plasma education program for European patient associations
In 2023, Grifols has promoted several educational initiatives with patient communities in Europe. Of particular note was a new edition of the “Plasma Awareness Education Program” which, among others, included specific update sessions on the new Substances of Human Origin (SoHO) regulation. The recently created European Alpha-1 Alliance is one of the patient organizations that participated in the program with 23 attendees and held its first General Assembly during the event.
- Community outreach
Grifols is committed to building trusting relationships, educating and supporting the patient communities it serves.
To reinforce this commitment, Grifols has its “Open House” educational program originally initiated in the United States and more recently also promoted in Europe. It includes giving participants a first-hand look at the production of plasma medicines at its facilities in Spain, Ireland and the U.S. and discussion on the issues impacting access.Participants include patient representatives of different patient associations.
- Raising awareness
In 2023, Grifols has brought the voice of patients to its employees by offering them the opportunity to see the impact of their daily work. Grifols’ professionals have been able to hear patient testimonials, in internal sessions, in forums such as IPAW (with inspiring patient stories in the two webinars organized) or onboarding and HR programs.
In addition, Grifols celebrated “Alpha-1 Month” to raise awareness of DAAT, which included visits to facilities in Barcelona (Spain) and Clayton (U.S.), among others.
