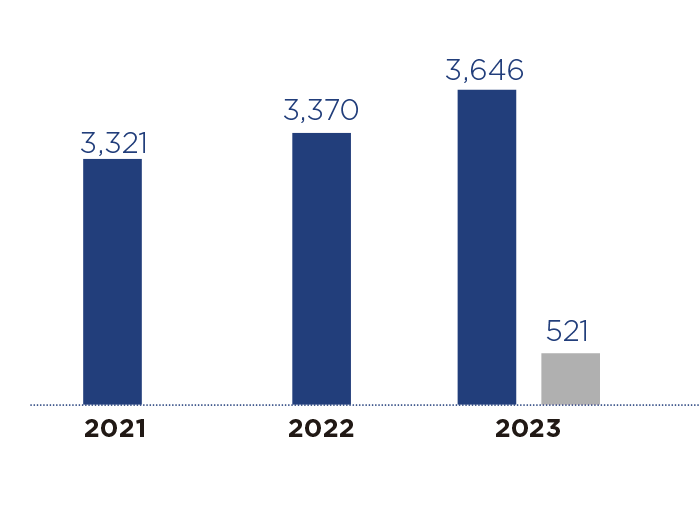Patients and healthcare professionals: relationships built on trust
Health, safety and pharmacovigilance measures
As outlined in its Quality Policy, Grifols identifies the critical attributes of its products and carries out exhaustive controls on the quality of raw materials, manufacturing processes, and finished product testing.
Grifols has pharmacovigilance agreements with all distributors, including those operating in countries with less advanced pharmacovigilance regulations, to ensure compliance with Grifols’ standards in this area.
The pharmacovigilance program monitors for any adverse effects or reactions resulting from its plasma-derived medicines, while its surveillance system detects adverse reactions stemming from the use of its medical and in vitro devices. Both programs feature systems to report safety issues and suspected cases of adverse reactions.
All activities and requirements of Grifols’ pharmacovigilance and surveillance systems are outlined in standard operating procedures and subject to routine reviews. The company conducts regular internal audits of these systems under its quality compliance protocols, which also undergo external inspections by the competent health authorities.
Grifols never outsources its pharmacovigilance and surveillance of medical and in vitro devices to third parties.
Packaging, leaflets and labeling
The information in Grifols’ product packages, leaflets and labels complies with the standards and regulations applicable in its countries of operation, the Good Manufacturing Practice (GMP) guidelines for pharmaceuticals, and country-specific regulations in other markets.
In terms of Grifols medical and in vitro devices, their labeling, instructions for the use of reagents, and instrument user and software manuals comply with country-specific regulations (EN ISO 15223, among others), and incorporate mitigating measures detected via medical-device risk management systems (EN ISO 14971 Medical Devices) or measures required by global health authorities. All printed material is translated to the corresponding language, updated as required and accessible to users.
Product recall system
The product recall system is governed by the corporate policy for patient and customer safety. Additionally, this system is developed through standardized work procedures and is internally audited by the company to verify its effectiveness and alignment with current regulations. It is also inspected by competent health authorities.
All Grifols teams involved in potential product recalls, whether voluntary or mandatory, receive specific training in proper incident management. Furthermore, Grifols conducts periodic product recall simulations to ensure that all crisis management procedures and protocols are functioning effectively and to identify any potential areas for improvement
The product claims and recall system includes procedures to notify healthcare authorities, patient associations and healthcare professionals regarding the potential risks of a recalled product. Grifols operates a customer service call center and has dedicated webpages for specific products to communicate potential risks. It also prohibits the use of any recalled product in clinical trials.
In 2023, Grifols did not have any mandatory product recalls due to quality or safety concerns. The company’s and Biotest voluntarily recalled two batches of products. Grifols’ stringent controls ensure comprehensive compliance with quality and safety standards.
Claims system
Grifols’ claims system, described in the corporate policy, registers and reviews all notifications received from healthcare centers, patients and users regarding consumer appraisals of possible quality issues. For medical devices, the management system for technical services is linked to the claims management system to ensure all client requests are evaluated.
When subsidiaries or authorized call centers receive a complaint regarding a Grifols medicinal product or service, they immediately notify the relevant production installation, ensuring all complaints are properly channeled and analyzed through the claims system.
The quality area of each business unit oversees the complaint process, which includes conducting the relevant investigations; verifying the implementation of corrective and preventive actions, if necessary; notifying relevant health authorities, if applicable; and informing the customer of the claim investigation’s findings.
 CLAIMS RATIO PER BUSINESS UNIT
CLAIMS RATIO PER BUSINESS UNIT
Biopharma
1 per 97,895
units distributed
2022: 1 per 77,806 units distributed
Diagnostic
1 per 559,298
diagnostic tests
2022: 1 per 482,302 diagnostic tests
Bio Supplies
1 per 2,777
units distributed
2022: No claims received
1 per 14,972,662
units distributed
2022: 1 per 5,848,478
units distributed
1 per 50,005
units distributed
2022: 1 per 31,210
units distributed
1 per 26,111
units distributed
2022: 1 per 32,532
units distributed
Counterfeit drug prevention system
Plasma medicines are prescription drugs that are primarily administered in hospital settings. As such, counterfeit products pose a grave risk to public health.
Grifols collaborates with regulatory authorities to investigate and analyze suspected cases of counterfeit, and has an internal policy to prevent, detect and report counterfeit products. In this regard, any suspicious and identified cases of counterfeit medicines must be duly and expeditiously reported to the relevant authorities in adherence to the applicable regulations in force.
Grifols uses track-and-trace technology to comply with product serialization and aggregation specifications required in certain countries and regions. These requirements include marking vials with a unique code before any plasma product is sold, and marking containers with a holographic seal to guarantee their inviolability and authenticity.
Grifols conducts routine internal audits and inspections to confirm regulatory compliance, and performs due diligence on customers and distributors to verify they possess the requisite licenses to distribute products. Its anti-counterfeiting measures are also detailed in third-party contracts and quality agreements when applicable.
Since 2021, Grifols is unaware of any actions resulting in raids, seizures, arrests and/or the filing of criminal charges related to counterfeit products.
More information: Grifols’ Anti-Falsification Policy
Responsible marketing practices
Grifols ensures its promotional and educational collateral complies with applicable laws and regulations; aligns with industry policies and voluntarily adopted codes; adequately addresses the target audience and end users; and contains truthful, accurate, comprehensive and balanced information.
The company has a standard operating procedure–the Grifols Review Process (GRP)–that specifies all activities and responsibilities related to the approval, review and control of promotional and educational materials used to communicate its products and services. Representatives from the legal, medical and regulatory departments review and approve all marketing collateral using a GRP-adapted electronic system. Marketing material and contents are solely approved for specific uses and countries, and may only be used with no alterations. The contents of all promotional and educational materials are regularly reviewed to confirm their validity and compliance with the standards and codes in force.
The company delivers appropriate training on responsible marketing and sales practices in line with its Code of Conduct and Anti- Corruption Policy.
In 2023, only one marketing complaint was received and handled according to established procedures. The complaint did not result in any monetary impact or loss.
Materials reviewed
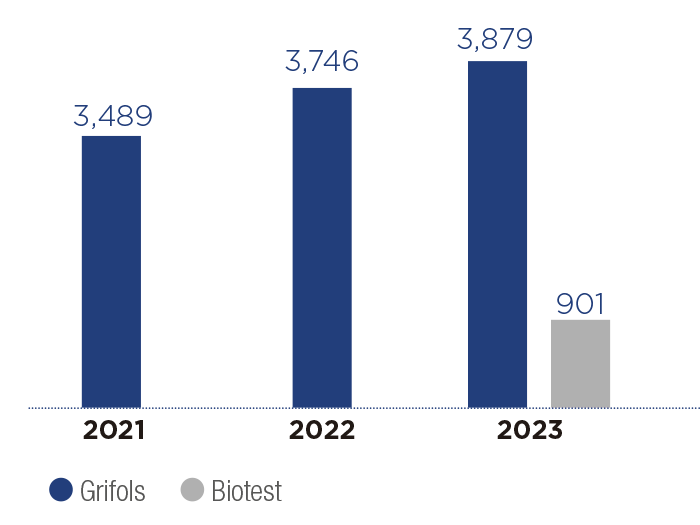
Materials approved
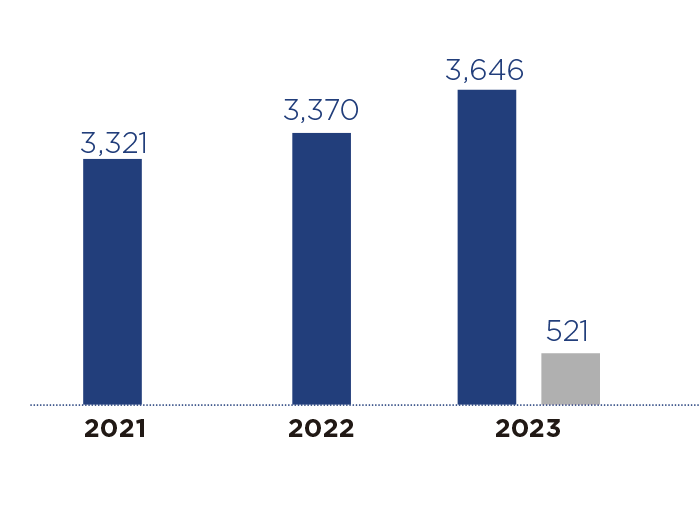
Overview of audits and inspections
PLASMA PROCUREMENT
Internal audits
256 (Grifols)
14 (Biotest)
Inspections by healthcare authorities and accredited inspection
organisms
529 (Grifols)
9 (Biotest)
Favorable
supplier audits
57 (Grifols)
4 (Biotest)
BIOPHARMA***
Internal audits
53 (Grifols)
20 (Biotest)
Inspections by healthcare authorities and accredited inspection organisms
22 (Grifols)
8 (Biotest)
Favorable supplier audits
139 (Grifols)
36 (Biotest)
DIAGNOSTIC
Internal audits
52
Routine inspections by
official institutions
14
Favorable supplier audits
28
OTHER****
Internal audits
76
Routine inspections by
official institutions
27
Favorable supplier audits
70
Incidents related to the suspension, revocation or loss of any license or certification; warning letter, imposed suspension of any regulated activity**
0
BIO SUPPLIES
Internal audits
2
Routine inspections by
official institutions
6
GOOD MANUFACTURING PRACTICES
* Includes inspections by health authorities and accredited inspection bodies, as well as in-house inspections.
** Includes Grifols and Biotest.
***Former Bioscience Division.
**** Others: includes Former Hospital Division.