The value of our collaborations
Advancing health care in three core areas
1. Public-private collaborations*:
We help countries bolster their plasma self-sufficiency to promote patients’ access to life-sustaining plasma-derived medicines.
2. Savings for healthcare systems:
We forge public-private partnerships that save costs for public healthcare systems.
3. Support for blood banks:
We work with blood bank to advance countries’ self-sufficiency of plasma-based medicines.
*More information: “Commitment to Donors and Patients”.
Optimizing health costs
Outside its core activity, Grifols shares its expertise in producing plasma medicines with other countries by making its facilities, technologies, knowledge and technical expertise available to public donation centers and health organizations. Grifols also processes surplus plasma, purifies the proteins, and returns to countries finished plasma-derived medicines.
These collaborations are offered in Spain, Italy and Canada under regulated by fractionation service agreements, leading to significant cost savings for these countries.
In 2023, this service was extended to Egypt as part of Grifols’ efforts to promote plasma self-sufficiency in the region.
![]()
€350+ M
in savings since 2019
GRIFOLS’ CONTRIBUTION TO HEALTH SYSTEM SAVINGS IN SPAIN
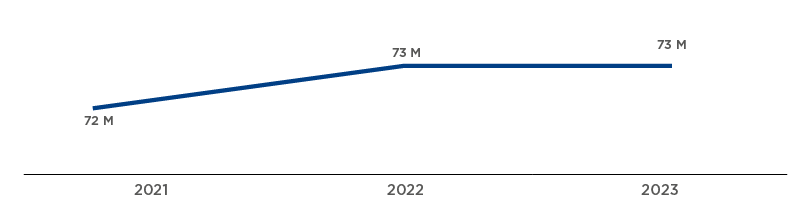
Spain boosts its plasma self-sufficiency for the production of plasma-based medicines
The procurement of human plasma–the essential raw material in the manufacture of plasma derivatives–has become a strategic priority for Spain’s National Health System. Throughout the year, actions were carried out to expand the plasma donor base and increase donations by apheresis.
In 2023, various groups joined forces to bolster plasma-collection volumes. For the third consecutive year, more than 400,000 liters of plasma were obtained for the fractionation and production of plasma derivatives, representing between 40% and 60% of Spain’s needs to produce plasma-based therapies.
Grifols joined the multiple awareness campaigns and actions organized in Spain to encourage plasma donations in line with its mission to enhance people’s health.
Collaborations with blood banks
International fractionation agreements
This broad-based service is customized to each client (public and private entities) and encompasses the entire plasma logistics chain (collection, transport, control and analysis) and its fractionation, purification, dosing and delivery of finished products.
The solution includes, among others, the Quality Program, which provides advice on quality management and assurance systems, the Academy Program, which includes training activities, courses, and programs related to plasm. At the same time, the Grifols Plasma Management Service web solution, was developed by Grifols to improve, streamline, and facilitate communication among the various parties involved in the industrial plasma fractionation contract monitoring and guaranteeing full traceability during the process.
 |
Collaborative solutions |
 |
Safety throughout the supply chain |
 |
Comprehensive quality control |
 |
Advancing countries’ plasma self-sufficiency |
 |
Patient-focused |
 |
Savings for healthcare systems |
Grifols spearheads several additional services to address the needs of blood banks and promote plasma self-sufficiency.
Additional services
- Apheresis Program: A collaboration with transfusion centers and blood banks to increase plasma donation by plasmapheresis. Through this initiative, Grifols offers its expertise to collaborating centers to develop educational and awareness-raising actions to encourage plasma donation by apheresis and increase plasma self-sufficiency.
- Contingency Program: Offers the center, in the event of an incident with its refrigeration equipment, the collection, temporary storage, and return of fresh frozen plasma.
- PROCLEIX® NAT Solutions: NAT technology tests enhance safety by reducing the risk of transfusion-transmitted diseases. They also improve laboratory efficiency with high test sensitivity.
- Biolab Program: This program provides several services:
- Viral Marker Sample Analysis using serology and NAT techniques.
- Confirmatory Testing for Doubtful Positives.
- Immunohematology Services.
- External Reference Laboratory Services.
- Quality Control for Fresh Frozen Plasma and Cryoprecipitate.
- Plasma Sample Storage and Management.
- Supply of Human Plasma for Various Tests or Controls.
- Biological Sample Archive: A service for controlled temperature (-80°C) storage, management, and delivery of biological samples.
